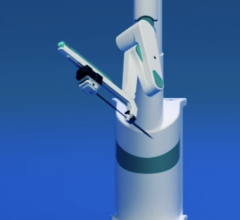
July 9, 2024 — Microbot Medical Inc. announced the completion of the first procedure in a patient utilizing its LIBERTY Endovascular Robotic Surgical System. The procedure took place at Brigham and Women’s Hospital (BWH), a leading academic medical center located in Boston, Massachusetts, as part of the company’s pivotal human clinical trial.
The clinical trial at BWH is led by Dr. Dmitry Rabkin, MD, PhD, Assistant Chief, Division of Angiography & Interventional Radiology, who also performed this first human case. The trial is part of the Investigational Device Exemption (“IDE”) for LIBERTY, and the company expects its results will support the future submission to the FDA and subsequent commercialization.
“Enrolling the first patient in our pivotal human clinical trial is a significant milestone for the company, and an important step on our journey to bring LIBERTY to U.S. physicians,” commented Juan Diaz-Cartelle, MD, Chief Medical Officer of Microbot Medical. “We are very pleased with the rapid pace of site activation, and I’m looking forward to enrolling additional patients in the near future.”
Microbot Medical Inc. is a clinical- stage medical device company that specializes in transformational micro-robotic technologies, with the goals of improving clinical outcomes for patients and increasing accessibility through the natural and artificial lumens within the human body.
The Investigational LIBERTY Endovascular Robotic Surgical System aims to improve the way surgical robotics are being used in endovascular procedures today, by eliminating the need for large, cumbersome, and expensive capital equipment, while reducing radiation exposure and physician strain. The Company believes the LIBERTY Endovascular Robotic Surgical System’s remote operation has the potential to be the first system to democratize endovascular interventional procedures.
For more information: www.microbotmedical.com


 January 27, 2026
January 27, 2026