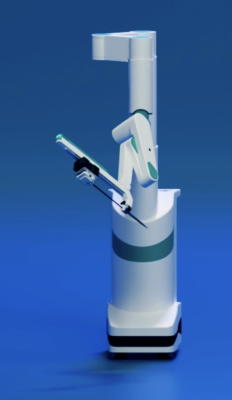
March 18, 2024 — SS Innovations International, Inc., a developer of innovative surgical robotic technologies dedicated to making world class robotic surgery affordable and accessible to a global population, today announced that surgeons have successfully performed the world’s first mitral valve replacement using the Made in India, SSi Mantra Surgical Robotic System. This procedure was carried out at the Narayana Hrudayalaya Institute of Cardiac Sciences in Bengaluru, India, and represents a milestone in the treatment of heart disease using the SSi Mantra Surgical Robotic System.
The mitral valve surgery was performed by Dr. Nitin Kumar Rajput from the Narayana Institute of Cardiac Sciences in Bengaluru, Karnataka, and guided by SS Innovations Founder and Chairman, Dr. Sudhir Srivastava, one of the early pioneers of robotic cardiac bypass surgery.
Considering this accomplishment, Dr. Srivastava commented, “We are very proud of our team for pushing the envelope to be able to add the field of full spectrum robotic cardiac surgery to all other surgical subspecialties. 90-95% of all cardiac surgeries are still done today using large sternum splitting incisions. Since the inception of the Company, it was always our goal to address the need of offering a highly minimally invasive solution for cardiac patients.
“During my practice in the US, I found that patients benefited immensely from surgical approaches which spared splitting of the sternum. Many patients would be discharged the following day and in full functional recovery in just a week to ten days later. The results were inspiring. This led me to develop more complex robotic bypass procedures that were adopted by other surgeons and would ultimately benefit more and more patients who underwent heart surgery.
“We are very proud that using our Made in India, SSi Mantra Surgical Robotic System, we have performed a variety of robotic heart surgeries including, Bilateral Internal Mammary Takedowns, Totally Endoscopic Coronary Artery Bypass on a beating heart, Atrial Septal Defect Repair and now a completely robotic Mitral Valve Replacement. I am very pleased that our original goal is now being met in conjunction with the highly talented surgeons at the Narayana Institute of Cardiac Sciences.”
Dr. Nitin Kumar Rajput, Consultant Cardiac Surgeon from Narayana Health, the primary surgeon on the Mitral Valve Replacement, commented following the successful completion of the procedure, “We have performed more than 60 CABGs (Heart Bypass Procedures) with the SSi Mantra Surgical Robotic System and we just did our first Robotic Mitral Valve Repair a few days back. The surgery went flawlessly, and it was wonderful operating with the Mantra System. Four ports were made for the robotic arm and a tiny 2.5 cm working port for the table side assistant. The surgery was completed quickly; the patient did well in the postoperative period and was discharged with good exercise tolerance three days post-surgery.”
Dr. Srivastava said: “SS Innovations is dedicated to improving patient access and optimizing surgical outcomes with our accessible and cost-effective surgical robotic system. We recognize the immense opportunity to address the significant unmet need for safe, timely, and affordable cardiac surgical care in India and around the world.”
“This milestone procedure is a testament to our commitment to advancing cardiac care. The successful operation opens new avenues for treating cardiac conditions, offering patients less invasive options, reduced recovery times, and better results.”
“The SSi Mantra embodies innovation and excellence and provides unmatched precision and control in robotic-assisted surgeries. Today's success is a proud moment for everyone at SS Innovations and our partners at Narayana Hrudayalaya,” concluded Dr. Srivastava.
The mitral valve disease market size was valued at $2.5 billion in 2021 and is estimated to reach $5.7 billion by 2031, growing at a CAGR of 8.8% from 2022 to 2031.
The SSi Mantra Surgical Robotic System, the first surgical robotic system to be made in India, and one of the few cost-effective global options with a wide range of surgical applications, has received regulatory approval in India, Indonesia and Guatemala, and is clinically validated for over 50 different types of surgical procedures. To date, more than 800 surgical procedures have been conducted using the system. SS Innovations has initiated the regulatory approval process in the United States and the EU, with approvals anticipated in the latter half of 2024 or 2025.
For more information: www.ssinnovations.com


 January 15, 2026
January 15, 2026