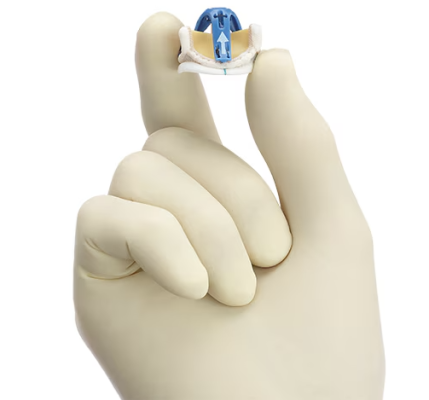
June 13, 2024 — Medtronic plc, a global leader in healthcare technology, today announced the launch of its latest innovation in cardiac surgery, the Avalus Ultra valve. This next-generation surgical aortic tissue valve is designed to facilitate ease of use at implant and lifetime patient management. It’s an excellent choice for cardiac surgeons and their patients seeking an aortic valve solution that can be fit for the future, right from the start.
The Avalus Ultra valve is engineered for ease of implant, clear visibility for future valve-in-valve procedures, and straightforward sizing. More specifically, the Avalus Ultra surgical valve has:
- A low valve profile designed to facilitate ease of use at implant.1,2
- A polyetheretherketone (PEEK) base frame, which provides consistent circularity3, 4
- An industry-leading Effective Orifice Area (EOA) that may allow for greater blood flow that is supported by the clinical evidence of the Avalus valve 5,6
- A radiopaque coil for clear visibility for future valve-in-valve procedures 7,8
Additionally, the Avalus Ultra valve builds on 10 years of clinical experience and the long-term durability of the Avalus valve.9,10,11
“Patients with aortic stenosis are experiencing more complex disease, and therefore, procedures need to evolve to optimize their surgical care,” said Professor Pieter Kappetein, vice president and chief medical officer of the Cardiac Surgery, Structural Heart, and Mechanical Circulatory Support businesses in the Medtronic Cardiovascular portfolio. “Rooted in this evolving need, surgeons are demanding innovative technologies like the Avalus Ultra valve that is designed for increased ease of use and long-term durability to serve patients over their lifetime.”
Aortic stenosis is a common heart problem typically caused by a narrowing of the heart’s aortic valve due to excessive calcium deposited on the valve leaflets. When the valve narrows, it does not open or close properly, making the heart work harder to pump blood throughout the body. Eventually, this causes the heart to weaken and function poorly, which may lead to heart failure and increased risk for sudden cardiac death.
A treatment for patients with aortic valve disease is surgical aortic valve replacement (SAVR). During this procedure, a surgeon will make an incision in the sternum to open the chest and expose the heart. The diseased native valve is then removed, and a new artificial valve inserted. Once in place, the device is sewn into the aorta and takes over the original valve’s function to enable oxygen-rich blood to flow efficiently out of the heart.
“Cardiac surgeons are looking to industry to partner with them to deliver improved innovation to treat their patients efficiently and effectively,” said Karim Bandali, Ph.D., president of the Cardiac Surgery business in the Medtronic Cardiovascular portfolio. “The Avalus Ultra valve builds on several successful innovation launches this year, including the acquisition and launch of the Penditure LAA Exclusion System. Aligned our Mission, Medtronic is committed to making the investments needed to advance and deliver innovative, lifesaving devices for better lifetime patient management.”
The Avalus Ultra valve received FDA approval in January 2024 and is currently only commercially available in the United States.
For more information: www.medtronic.com
References:
1 Based on internal test report D00998354, Avalus Ultra HFE Design Validation Test Report.
2 Based on internal document D00437207, Avalus Ultra Design Concept.
3 Based on internal test report D00997823, Avalus Ultra Full Valve Stiffness Design Verification Report.
4 Based on internal test report D00998399, Design Characterization Report: External Sewing Ring Diameter, Valve Housing External Diameter, and Inflow Orifice Diameter of Avalus Ultra.
5 Verbelen T, Roussel JC, Cathenis K, et al., Real-world data on the Avalus™ pericardial aortic valve: initial results from a prospective, multi-center registry. Presented at Heart Valve Society 2024, Boston, MA. February 18–21, 2024.
6 Klautz RJM, Dagenais F, Reardon MJ, et al. Surgical aortic valve replacement with a stented pericardial bioprosthesis: 5-year outcomes. Eur J Cardiothorac Surg. August 2022;62(3):ezac374.
7 Based on internal test report D00998354, Avalus Ultra HFE Design Validation Test Report.
8 Based on internal document D00437207, Avalus Ultra Design Concept
9 Klautz RJM, Dagenais F, Reardon MJ, Lange R, Moront MG, Labrousse L et al. Surgical aortic valve replacement with a stented pericardial bioprosthesis: 5-year outcomes. Eur J Cardiothorac Surg 2022 Aug 3;62(3)
10 Klautz RJM, Rao V, Reardon MJ, et al. Hemodynamic function of contemporary surgical aortic valves 1 year postimplant. Abstract presented at: 37th Annual Meeting of the European Association for Cardio-Thoracic Surgery; October 4-7, 2023; Vienna, Austria.
11Sohn SH, Kang Y, Kim JS, et al., A Randomized Trial Comparing One-year Hemodynamics of Two Bovine Pericardial Valves. Thorac Cardiovasc Surg. 2023 Dec 5


 December 24, 2025
December 24, 2025 









