July 25, 2024 — BioCardia, Inc., a global leader in cellular and cell-derived therapeutics for the treatment of cardiovascular and pulmonary diseases, announced today that the confirmatory Phase 3 trial of its autologous CardiAMP cell therapy product candidate for patients with ischemic heart failure of reduced ejection fraction (HFrEF) has commenced enrollment in the United States.
BioCardia previously confirmed alignment with the United States Food and Drug Administration (FDA) on the design of the 250-patient randomized, controlled trial and the minimum of 12-month primary composite endpoint of all cause death, reduction in major adverse cardiovascular events, and improvement in quality of life. FDA granted CardiAMP Cell Therapy a Breakthrough Device Designation for the treatment of ischemic heart failure. Breakthrough designation provides for FDA to expedite development and prioritize review of regulatory submissions intended to help patients have more timely access to these product candidates.
“The CardiAMP cell therapy has the potential to be groundbreaking and life changing for patients with heart failure,” said Dr. Leslie Miller, MD, trial investigator at the CHF Heart Function Clinic at BayCare Morton Plant Hospital in Clearwater, Florida and a member of the CardiAMP Heart Failure II Study Executive Steering Committee. “As the principal investigator of the first site activated to enroll patients in this important study, I have identified many patients who may benefit from inclusion in this clinical trial, including nine patients in my clinic in active screening to participate after meeting the important NTproBNP inclusion criterion.”
BioCardia Chief Executive Officer, Peter Altman, PhD said “We are looking forward to expeditious enrollment in the trial ahead working with world class clinical heart function teams with the objective of confirming the trends of enhanced survival, reduced major adverse events, and improved quality of life observed in previous studies. There is a significant need for a safe, effective, and durable treatment for patients with ischemic heart failure, in particular one that positively impacts patient survival and quality of life.”
About Chronic Heart Failure of Reduced Ejection Fraction (HFrEF)
Heart failure of reduced ejection fraction (HFrEF) is a clinical condition in which the output of blood from the heart is insufficient to meet the metabolic demands of the body. In 2022, the American Heart Association report on heart disease statistics estimated that 3 million American adults ages 20 and older have heart failure of reduced ejection fraction with a prevalence expected to increase to more than 4 million people by 2030. During heart failure progression, the heart steadily loses its ability to respond to increased metabolic demand, and mild exercise soon exceeds the heart’s ability to maintain adequate output. Towards the end stage of the disease, the heart cannot pump enough blood to meet the body’s needs at rest. At this stage, fluids accumulate in the extremities or in the lungs making the patient bedridden and unable to perform the activities of daily living.
Despite guideline-directed therapies employing a wide range of pharmacologic, device, and surgical options, many patients deteriorate over time and develop advanced heart failure symptoms that cannot be effectively managed by existing medical therapies.
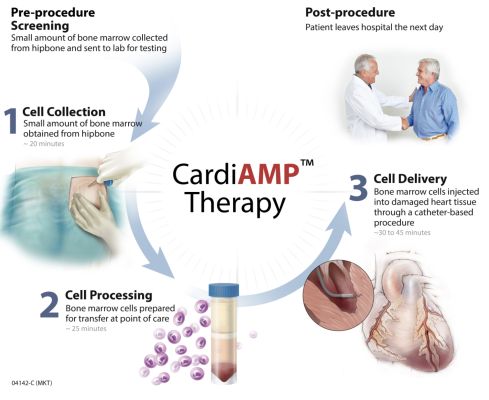
About CardiAMP Cell Therapy
Granted Breakthrough Designation by the FDA, the CardiAMP Cell Therapy uses a patient’s own bone marrow cells delivered to the heart in a minimally invasive, catheter-based procedure to potentially stimulate the body’s natural healing response. CardiAMP Cell Therapy incorporates three proprietary elements not previously utilized in investigational cardiac cell therapy: a pre-procedural cell analysis for patient selection, a high target dosage of cells, and a proprietary delivery system that has been shown to be safer than other intramyocardial delivery systems and exponentially more successful in cell retention. Clinical development to date in randomized controlled double blinded trials has shown trends towards enhanced patient survival, reduced major adverse cardiac events, and improved quality of life. The CardiAMP clinical development for heart failure is supported by the Maryland Stem Cell Research Fund and is reimbursed by Center for Medicare and Medicaid Services (CMS) for both treatment and control procedures. CAUTION - Limited by United States law to investigational use.
About CardiAMP Heart Failure Clinical Development
BioCardia expects final study results from the randomized double blinded controlled one hundred and twenty-five subjects CardiAMP Heart Failure I Study in November 2024. Interim results demonstrated a 37% relative risk reduction in all cause death with 90% of the follow-up data available. BioCardia’s clinical team is actively working with investigational sites performing source data verifications with the goal of sharing final results with both the FDA and Japan’s Pharmaceutical and Medical Device Agency.
The confirmatory CardiAMP Heart Failure II study focuses on patients in active heart failure who demonstrated the greatest benefits in the interim results of the CardiAMP Heart Failure I study. This subgroup of patients showed strong signals of benefit with 86% relative risk reduction in mortality and the primary outcome measure approaching statistical significance at two years.
The CardiAMP Heart Failure II study also uses a validated quality of life patient self-assessment instrument as the third component of the primary endpoint instead of the distance walked in six minutes. This endpoint would have been statistically significant in the patients with active heart failure that are the focus of the CardiAMP Heart Failure II study (p=0.03). The study design has > 90% power or probability of demonstrating statistical significance based on the CardiAMP HF I interim results.
The world class cardiologists who comprise the Co-National principal investigators, Executive Steering Committee Members, Clinical Events Committee and the Data Safety Monitoring Board from the CardiAMP Heart Failure I study are all continuing with the CardiAMP Heart Failure II study with world class additions. Many clinical sites are in active stages of being onboarded and activated.
For more information: www.biocardia.com


 February 03, 2026
February 03, 2026 









