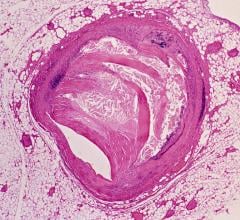
June 14, 2019 – A late-breaking study examined the effects of intravascular ultrasound (IVUS) guided drug-eluting stent (DES) implantation on patients with chronic kidney disease (CKD). Results showed that CKD patients treated with IVUS-guided DES implantation had better outcomes compared to those who received the traditional angiography guidance DES implantation at 12 months. The prespecific subgroup analysis of the prospective, multicenter, randomized ULTIMATE Trial was presented at the Society for Cardiovascular Angiography and Interventions (SCAI) 2019 Scientific Sessions in May.
Chronic kidney disease is a growing worldwide health issue affecting one in 10 individuals, according to the National Kidney Foundation. Patients with CKD are more than 30 percent likely to have cardiovascular disease which is the leading cause of death, according to the National Institutes of Health (NIH). While several randomized trials and observational studies have established the clinical benefits of IVUS-guided DES implantations for patients with complex lesions, the treatment option remains controversial for patients with CKD due to longer procedural time and perceived potential risks of acute renal failure and atheroembolism.
“The present study, which was a prespecified subgroup analysis of the ULTIMATE trial, was designed to explore the impacts of IVUS-guided second-generation DES implantations on patients with CKD.” said Junjie Zhang, M.D., Ph.D., Nanjing First Hospital, Nanjing Medical University, China. “The results from our study show that compared to angiography guidance procedures, IVUS-guided DES implantation is a new and effective treatment approach. This procedure has the potential to significantly decrease target vessel failure in CKD patients and, ultimately, improve their quality of life.
The study assessed 1,443 patients of whom 723 underwent IVUS-guided DES implantations and 720 underwent angiography-guided DES implantations from August 2014 to May 2017. The inclusion criteria were patients who had silent ischemia, stable or unstable angina, or myocardial infarctions from the onset of chest pain to admission, as well as de novo coronary lesions that were eligible for DES implantation. The trial was conducted in eight hospitals throughout China and was approved at each participating center. CKD was present in 349 (24.2 percent) patients. CKD patients were older and more frequently presented with a history of stroke, hypertension, symptomatic heart failure (HF), and lower left ventricular ejection fraction (LVEF).
At 12 months, the target vessel failure (TVF) in the CKD group was 7.2 percent, significantly higher than 3.2 percent in the non-CKD group, which was mainly driven by increased risk of cardiac death in CKD patients, compared to non-CKD patients (2.9 vs. 0.5 percent). Moreover, there were 25 TVFs in CKD patients, with seven TVFs (3.9 percent) in the IVUS group and 18 TVFs (10.7 percent) in the angiography group. The reduced risk of TVF in the IVUS group for CKD patients was mainly driven by the lower risk of Target Vessel MI (TVMI) (0.6 vs. 3.6 percent) and Target Vessel Revascularization (TVR) (1.1 vs. 4.7 percent).
To further assess long-term efficacy of IVUS-guided DES implantation as a treatment option, the authors of this study call for a broader randomized trial.
Watch a VIDEO interview with Zhang on this trial from TCT 2018.
For more information: www.scai.org


 October 24, 2025
October 24, 2025