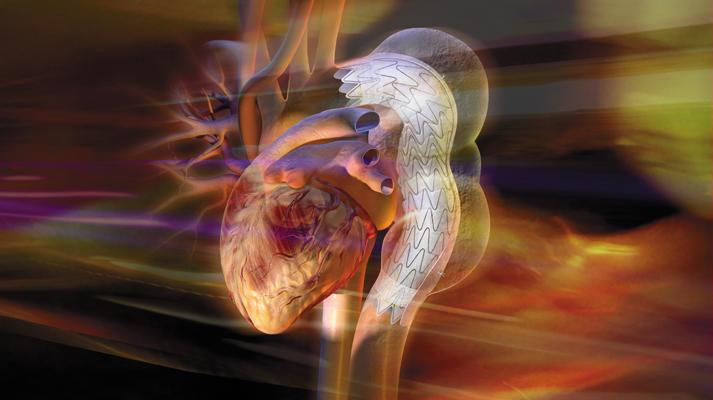
May 5, 2014 — W. L. Gore & Associates (Gore) announced that Himanshu Patel, M.D. and David Williams, M.D. at The University of Michigan enrolled the first patient in the Gore TAG Thoracic Branch Endoprosthesis LSA Feasibility Study, a U.S.-based, multi-center feasibility trial. The Food and Drug Administration (FDA) approved the investigational device exemption (IDE) trial for the Gore TAG Thoracic Branch Endoprosthesis in the treatment of thoracic aortic aneurysms that require coverage of the left subclavian artery (LSA).
“Thoracic aortic aneurysms that encroach on the LSA make treating these challenging anatomies very difficult, leaving physicians no choice but to use more invasive surgical techniques or to cover the branch vessel,” said Patel. “Using the Gore TAG Thoracic Branch Endoprosthesis, we were able to successfully treat the first patient in the study using endovascular means only. These results demonstrate the feasibility of using branched stent-grafts to treat aortic aneurysms that involve the LSA.”
“With this study, we hope to demonstrate that the Gore device allows physicians to safely treat aortic aneurysms near the aortic arch while maintaining blood flow to all branch vessels using endovascular techniques,” said Michael Dake, M.D., principal investigator and Thelma and Henry Doelger Professor of Cardiovascular Surgery at the Stanford School of Medicine, Stanford, Calif. “Gore’s device will reduce the need for invasive surgical procedures commonly required today, thus reducing the complications associated with treatment of this complex disease.”
The Gore TAG Thoracic Branch Endoprosthesis builds on more than 15 years of Gore experience in aortic innovation. Designed for long-term durability, the device allows for femoral-only access over a pre-positioned branch guidewire for ease of implantation. The device also features the CARMEDA BioActive Surface (CBAS Heparin Surface) for sustained anti-thrombotic bioactivity, as is present on many of Gore’s peripheral vascular products.
Gore accomplished several key regulatory milestones in 2013. The Gore Excluder Iliac Branch Endoprosthesis received CE mark in October 2013 and is currently available for investigational purposes only in the U.S. Additionally, the FDA approved the Conformable Gore TAG Thoracic Endoprosthesis for the treatment of acute and chronic dissections, making it the first and only stent-graft that is FDA approved to treat aneurysms, traumatic transections, and dissections of the thoracic aorta.
For more information: www.gore.com


 January 05, 2026
January 05, 2026 









