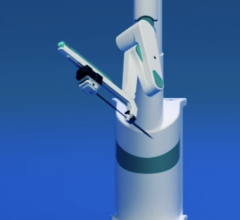
June 20, 2024 — Microbot Medical Inc. announced its agreement with Brigham and Women’s Hospital (BWH), a leading academic medical center located in Boston, Massachusetts, to serve as one of the sites to perform the pivotal human clinical trial for its LIBERTY Endovascular Robotic Surgical System, as part of its Investigational Device Exemption (“IDE”) application.
This development, previously announced on June 17, 2024, follows the U.S. Food and Drug Administration’s approval to commence Microbot’s pivotal human clinical trial.
The Company has completed the Site Initiation Visit, during which BWH clinical staff was trained on the clinical study protocols and the use of the LIBERTY Endovascular Robotic Surgical System. In addition, the first shipment of LIBERTY investigational systems arrived at BWH this week in support of the clinical trial. Dr. Dmitry Rabkin, MD, PhD (Assistant Chief, Division of Angiography & Interventional Radiology), will lead the study for the site as principal investigator at BWH.
“We are pleased to work with Dr. Rabkin and the team at Brigham and Women’s Hospital on this clinical study,” commented Harel Gadot, CEO, President and Chairman of Microbot Medical. “We believe their commitment to research and the advancement of science make them an ideal clinical study site.”
The Company is in the process of engaging additional leading centers to participate in the clinical trial.
For more information: www.microbotmedica.com


 January 27, 2026
January 27, 2026