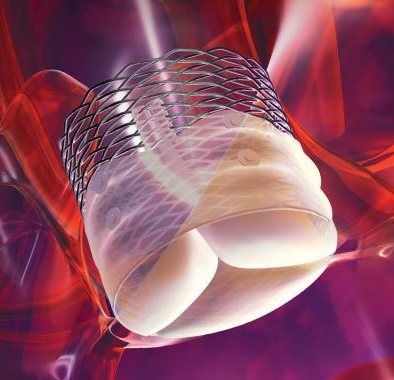
May 29, 2013 — The Lotus Valve, a second-generation transcatheter aortic valve implantation (TAVI) device, was successfully implanted in all of the first 60 patients in results from REPRISE II, which showed good device performance and low mortality at 30 days.
"First generation TAVI devices provide significant clinical benefit, but there are opportunities for improvement," said lead author Ian Meredith, director of MonashHeart, Southern Health and professor of medicine, Monash University, Melbourne, Australia. He suggested that these include controlled deployment, simple, precise and atraumatic aortic/ventricular repositioning, no or trivial paravalvular leakage and lower complication rate.
The Lotus Valve System has been designed to address these issues. The valve is pre-attached to the delivery system, which has a simple handle design, and functions early in deployment for controlled, precise positioning. It is fully retrievable and can be repositioned and has an adaptive seal designed to minimize paravalvular leak.
REPRISE II prospectively evaluated the safety and performance of the Lotus Valve System for TAVI in symptomatic patients with severe calcific aortic stenosis considered high risk for surgical valve replacement. The primary endpoint for device performance — mean aortic valve pressure gradient at 30 days compared to a performance goal of 18 mmHg — was met. Mean aortic gradient decreased from 47.5+17.2 mmHg before the procedure to 11.3+5.2 mmHg at 30 days. At the same time effective orifice area increased from 0.6+0.2 mmHg to 1.7+0.4 mmHg.
"Results showed successful valve implantation in all 60 patients, meeting the primary device performance endpoint," Meredith said. "Importantly, the rate of moderate or greater aortic regurgitation decreased from 18 percent at the baseline study to 1.9 percent (one patient) at 30-day follow-up. More than 80 percent had either no or trivial aortic regurgitation at 30 days," he reported. He pointed out that the very low rate of moderate or greater aortic regurgitation was 10-fold lower than seen in previous trials with other systems, describing this as 'a monumental improvement. The mortality rate was low — 1.7 percent at 30 days.
"Valve repositioning and retrieval was performed successfully in all cases when required. And there was no embolization, ectopic valve deployment or TAV-in-TAV," he added. Patients suffered negligible aortic regurgitation, while other clinical event rates were consistent with those reported for other valves.
For more information: www.escardio.org


 January 05, 2026
January 05, 2026 









