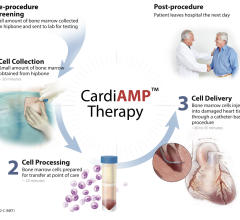
Impulse Dynamics Optimizer Smart for heart failure
April 4, 2022 – Impulse Dynamics, a global medical device company dedicated to improving the lives of people with heart failure (HF), today announced that the first patient has been enrolled in the quadruple-blinded sham-controlled AIM HIGHer IDE clinical study (Assessment of Implantable CCM in the Heart Failure Group with Higher Ejection Fraction) evaluating CCM therapy for patients who have left ventricular ejection fraction ranging between 40% and 60% inclusive. The study will be conducted across approximately 150 centers worldwide.
Simos Kedikoglou, MD, CEO of Impulse Dynamics, said, “AIM HIGHer is another example of our continuous investment in CCM therapy for the benefit of HF patients. Impulse Dynamics is undertaking extensive product and clinical development to bring hope to HF patients worldwide and help them lead more comfortable and productive lives.”
“AIM HIGHer is a remarkable study in several respects,” said Ishu Rao, MD, Medical Director for Impulse Dynamics. “Not only is this the largest randomized sham-controlled therapeutic cardiac device trial to ever be conducted, but it’s studying a label expansion for a new, large, and quite different patient population. These patients have few effective options for treating their condition, and this study is an important step towards offering a solution to this significant unmet medical need.”
HF affects more than 64 million people worldwide and leads to dramatic declines in the quality of life. Patients are often classified by a measure of cardiac function known as the ejection fraction (EF), which describes the percentage of blood pumped out of the left ventricle with each heartbeat. Patients can have HF with severely reduced, moderately reduced, or even preserved ejection fraction (e.g., EF close to the normal range of 60% or above.) Those patients with severely reduced or moderately reduced EF have therapeutic options, including many classes of pharmaceuticals and device therapies such as CCM. However, HF patients with higher EF (e.g., above 40%) have had few therapeutic options thus far to alleviate their symptoms and treat their disease. The purpose of AIM HIGHer is to assess the potential of CCM to improve performance and reduce cardiovascular morbidity and mortality for these patients.
Nareg Minaskeian, PhD, MD, a cardiac electrophysiologist with Southwest Cardiovascular Associates in Mesa, AZ who conducted the first AIM HIGHer implant procedure remarked, “We’re already using CCM therapy for HF patients who have reduced ejection fraction with excellent results, and we have a great deal of hope that CCM will also prove to be effective in patients with a higher EF.”
Javed Butler, MD, MPH, MBA, President of Baylor Scott & White Research Institute, SVP of Baylor Scott & White Health System in Dallas, TX, Distinguished Professor of Medicine at the University of Mississippi and Primary Investigator for the AIM HIGHer trial commented, “It is exciting to have this important trial move from planning to execution phase. This is a high-risk group of patients without many good therapeutic options. CCM therapy is a promising approach to treat these patients, and the AIM HIGHer trial will provide much-needed evidence in this area.”
Dr. Oussama Wazni, Section Chief of Electrophysiology at the Cleveland Clinic and Co-Primary Investigator of AIM HIGHer, was similarly enthused: “We are pleased to begin the AIM HIGHer trial and commend Dr. Nareg Minaskeian on implanting the first device as part of the study. This trial will evaluateCCM therapy in heart failure patients with an EF over 40% — a group that currently has few treatment options.”
The Optimizer delivers cardiac contractility modulation therapy — a proprietary technology of Impulse Dynamics — to the heart. Impulse Dynamics has designed CCM therapy to significantly improve heart contraction, allowing more oxygen-rich blood to be pushed out through the body.1 This breakthrough device has demonstrated its capability of enhancing the quality of life for HF patients no longer responding adequately to medication meant to manage their symptoms.1 CCM therapy is indicated to improve a six-minute hall walk, quality of life, and functional status of NYHA Class III HF patients who remain symptomatic despite guideline-directed medical therapy, who are not indicated for CRT and have a left ventricular ejection fraction ranging from 25% to 45%.
For more information: impulse-dynamics.com/
Reference:
1 European Journal of Heart Failure (2021) doi:10.1002/ejhf.2202


 November 18, 2024
November 18, 2024