December 16, 2009 – Patient enrollment was recently completed for a study evaluating the ability of cardiopoietic cells to restore cardiac function in heart failure patients.
Cardio3 BioSciences said this week it completed enrollment two months ahead of schedule for the first stage of its pivotal phase II/III trial of C-Cure, a stem cell therapy for heart failure. Forty-five patients will undergo treatment at centers in Belgium and Serbia. The C-Cure trial will ultimately enroll 240 patients, making it one of the largest randomized trials in regenerative therapies for heart failure.
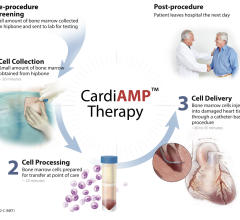
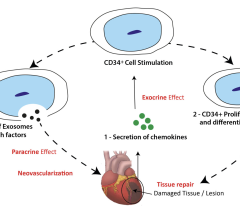
C-Cure is produced by taking a patient’s own bone marrow cells and, through a proprietary process, differentiating them into cardiopoietic cells that can regenerate damaged heart muscle. The cardiopoietic cells are injected into the heart of a patient with heart failure where they are designed to behave identically to those cells lost in heart failure without carrying the risk of rejection, something that has not been achieved with previous cell therapies for this indication. C-Cure is the outcome of multiple years of research conducted at Mayo Clinic and the Cardiovascular Center in Aalst, Belgium.
Safety data from this stage of the trial is expected to be available in May 2010. The second stage, which will recruit 195 further patients, is expected to start in the third quarter of 2010 and involve further sites in Europe and in the United States. The trial design is a randomized, prospective, multicenter trial to evaluate the safety and efficacy of C-Cure beyond optimal clinical care in patients with heart failure. The trial will also evaluate socio-economic implications of therapy.
For more information contact: www.c3bs.com


 November 18, 2024
November 18, 2024