April 17, 2012 - On April 9, 2012, the FDA's Center for Devices and Radiological Health (CDRH) launched its second ...
Diagnostic and Interventional Cardiology DAIC
Diagnostic and Interventional Cardiology (DAIC) is a magazine that reaches more than 25,000 healthcare professionals in cardiology, interventional cardiology and cath labs across the United States. These influential buying team members rely on DAIC's award-winning editorial content and comparison charts as a unique research tool for specifying, recommending and approving technology/device purchases.
April 17, 2012 – The U.S. Food and Drug Administration (FDA) has approved a humanitarian use device (HUD) designation ...
April 12, 2012 - Abiomed Inc., a provider of heart support technologies, announced it has received CE marking approval ...
April 12, 2012 – Terumo Interventional Systems recently announced the nationwide availability of the Azur D35 Framing ...
April 12, 2012 — Philips Healthcare recently installed an Allura Xper FD 20/20 biplane angiography X-ray system at ...
April 12, 2012 - Edwards Lifesciences Corp. said a U.S. Food and Drug Administration (FDA) advisory panel will review ...
April 12, 2012 – The U.S. Food and Drug Administration (FDA) this week approved an expanded indication for Medtronic’s ...
April 11, 2012 — ReCor Medical disclosed updated data for the REDUCE First-In-Man clinical study of its CE-marked ...
April 9, 2012 — Thoratec and the U.S. Food and Drug Adminstration (FDA) announced a recent Class 1 recall of the ...
April 6, 2012 — Crux Biomedical announced that a recently completed pivotal trial of its Crux Vena Cava Filter (VCF) ...
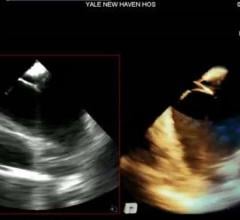
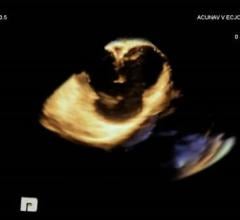
Siemens' AcuNav V 3-D intracardiac echo (ICE) catheter offers detailed, live 3-D images of the interior of the heart ...
Siemens' AcuNav V 3-D intracardiac echo (ICE) catheter offers detailed, live 3-D images of the interior of the heart ...
Siemens' AcuNav V 3-D intracardiac echo (ICE) catheter offers detailed, live 3-D images of the interior of the heart ...
April 4, 2012 — Medtronic Inc. announced that the Symplicity renal denervation system provides safe, significant and ...
April 4, 2012 — Edwards Lifesciences Corp. reported that longer-term results (? 2 years) from the high-risk Cohort A of ...

 April 17, 2012
April 17, 2012