January 18, 2013 — CardioMEMS Inc. was honored as the 2012 recipient of the Intel Innovation Award. Founder and CEO Jay ...
Diagnostic and Interventional Cardiology DAIC
Diagnostic and Interventional Cardiology (DAIC) is a magazine that reaches more than 25,000 healthcare professionals in cardiology, interventional cardiology and cath labs across the United States. These influential buying team members rely on DAIC's award-winning editorial content and comparison charts as a unique research tool for specifying, recommending and approving technology/device purchases.
January 18, 2013 — CardioKinetix Inc. announced the enrollment of the first patients in PARACHUTE IV, the randomized ...
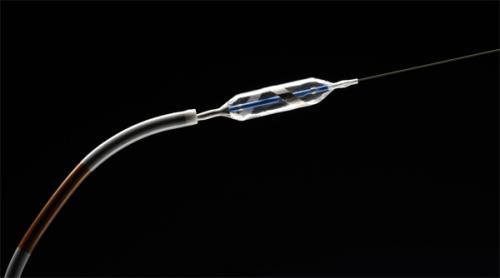
January 16, 2013 — Covidien began the commercial launch of the OneShot Renal Denervation System, an over the wire ...
January 16, 2013 — Open heart surgery may be a dying art, as new valve replacement techniques offer life-changing ...
January 16, 2013 — CardioLogical Solutions, a new cardiovascular device company, announced it has initiated operations ...
January 16, 2013 — Rex Medical L.P. announced that it has received 510(k) Clearance from the U.S. Food and Drug ...
January 8, 2013 — CardiAQValve Technologies Inc. received $37.3 million in funding in its second round of equity ...
January 3, 2013 — BioVentrix announced it has received CE mark for its Revivent myocardial anchoring system, which makes ...
January 3, 2013 — The Society of Cardiovascular Computed Tomography (SCCT) announced the publication of the SCCT Expert ...
December 31, 2012 — According to Millennium Research Group (MRG), a company focused on global medical technology market ...

December 28, 2012 — The past year in cardiology has continued to see progress and innovation in medicine. As the year ...
December 28, 2012 — Resuscitation, cell regeneration, a new high blood pressure treatment and developments in devices ...
December 27, 2012 — Cardiosonic Inc., a private developer of technology in the field of renal denervation for the ...
December 27, 2012 — According to Millennium Research Group (MRG), approximately 80 percent of transcatheter aortic valve ...
The annual Transcatheter Cardiovascular Therapeutics (TCT) meeting brings many cutting-edge technologies to the ...

 January 18, 2013
January 18, 2013


