April 12, 2013 — A research study of more than 600 black patients with uncontrolled hypertension found that less than ...
Diagnostic and Interventional Cardiology DAIC
Diagnostic and Interventional Cardiology (DAIC) is a magazine that reaches more than 25,000 healthcare professionals in cardiology, interventional cardiology and cath labs across the United States. These influential buying team members rely on DAIC's award-winning editorial content and comparison charts as a unique research tool for specifying, recommending and approving technology/device purchases.
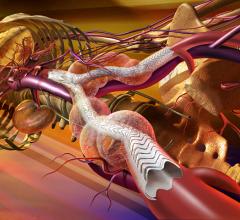
April 12, 2013 — NeoChord has received CE mark for its technology that allows the implantation of artificial chordae ...
April 10, 2013 — MIM Software Inc. has announced its software release MIM 6 is now available. Users in all areas of ...
April 8, 2013 — W. L. Gore & Associates Inc. has received U.S. Food and Drug Administraion (FDA) approval for the new ...
April 8, 2013 — Angiotech Pharmaceuticals Inc. announced that it entered into a definitive agreement to sell certain of ...
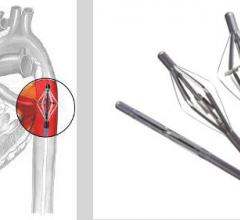
April 5, 2013 — Continuing its commitment to provide best-in-class medical devices for the prevention of recurrent ...
April 3, 2013 — BioControl Medical has received U.S. Food and Drug Administration (FDA) approval to begin the third and ...
April 3, 2013 — An RTI International-developed prototype catheter that can generate live, streaming 3-D ultrasound ...
April 1, 2013 — Rox Medical announced enrollment of the first U.K. patients in the CONTROL-HTN international randomized ...
March 28, 2013 — Thoratec Corp. announced that it has successfully completed the first human use of HeartMate PHP ...
March 28, 2013 — St. Jude Medical announced publication of results from its landmark RESPECT clinical trial in The New ...
March 27, 2013 — The Heart Failure 2013 LA meeting will include a showcase of several startup companies offering ...
March 26, 2013 — A multicenter analysis, led by Weill Cornell Medical College and published in the Journal of Clinical ...
March 25, 2013 — In addition to improving life quality and diminishing pain, total hip replacement (THR) is associated ...
March 25, 2013 — W. L. Gore & Associates introduced its Gore DrySeal Sheath with hydrophilic coating in Europe. The new ...


 April 12, 2013
April 12, 2013