Diagnostic and Interventional Cardiology DAIC
Diagnostic and Interventional Cardiology (DAIC) is a magazine that reaches more than 25,000 healthcare professionals in cardiology, interventional cardiology and cath labs across the United States. These influential buying team members rely on DAIC's award-winning editorial content and comparison charts as a unique research tool for specifying, recommending and approving technology/device purchases.
November 15, 2013 – Neovasc Inc. announced that the first patent covering the company's innovative Tiara transcatheter m ...
The RSNA FastPass is produced by Imaging Technology News (ITN) as a guide to what vendors are highlighting. It helps ...
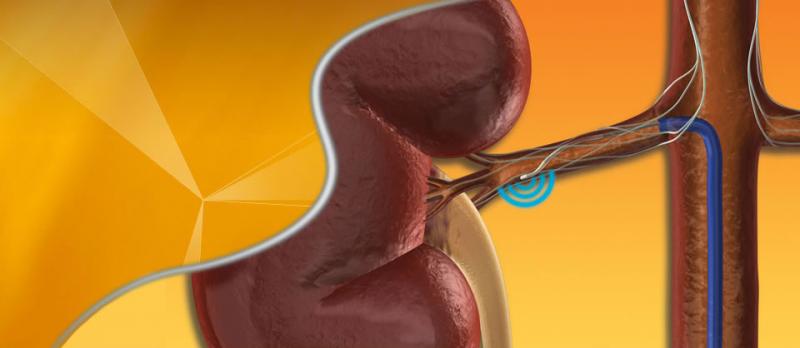
By Dave Fornell, DAIC editor
The Cardiovascular Research Foundation’s Transcatheter Cardiovascular Therapeutics (TCT) ...

November 8, 2013 – According to Millennium Research Group (MRG), the anticipated Food and Drug Administration (FDA) ...
Scott Lim, M.D., director of the Heart Valve Center at the University of Virginia Medical Center, investigator in the ...


 November 18, 2013
November 18, 2013








