August 30, 2013 — PFM Medical received U.S. Food and Drug Administration (FDA) premarket approval (PMA) for its Nit-Occlud Patent Ductus Arteriosus (PDA) device. Nit-Occlud PDA is a permanently implanted prosthesis indicated for percutaneous, transcatheter closure of small to moderate size patent ductus arteriosus with a minimum angiographic diameter less than 4 mm.
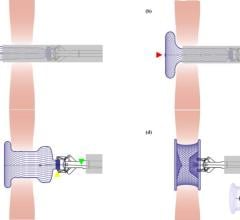
A catheter containing the occluder is threaded into a vein in the groin and through the pulmonary artery. It is then advanced through the defect. When it is in the correct position, the coil is advanced through the catheter into the aorta. The coil wire is then released so that the last loop is on the pulmonary side of the defect.
The Nit-Occlud PDA device functions as a “plug” (occluder) to close an abnormal opening between the pulmonary artery and the aorta. The occluder is made of a self-expanding coil spiral that consists of an inner (core) wire tightly wrapped by an outer coil wire. After the device is in place, tissue will grow over it and the device then becomes part of the pulmonary artery.
This device can prevent blood from passing through the PDA, which may cause symptoms such as fatigue, difficult or rapid breathing, failure to grow normally, or chronic respiratory infections such as colds and pneumonia. Large openings can lead to heart failure and death. The device is implanted without the use of open-heart surgery.
A Phase II clinical study was conducted with 378 subjects enrolled in 15 study centers throughout the United States under an investigational device exemption (IDE) to establish a reasonable assurance of safety and effectiveness of the Nit-Occlud PDA. Favorable results meeting all objective performance criteria from this clinical study were the basis for the premarket approval decision.
For more information: www.pfmmedical.com


 June 20, 2024
June 20, 2024