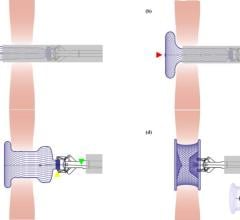
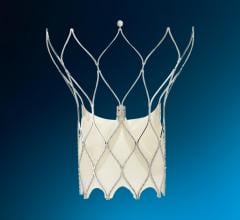
The Abbott Amplatzer Talisman PFO Occlusion System is designed to treat people with a patent foramen ovale (PFO). The small opening between the upper chambers of the heart can increase the risk of recurrent ischemic stroke.
October 4, 2021 — U.S. Food and Drug Administration (FDA) has cleared the Abbott Amplatzer Talisman PFO Occlusion System to treat people with a patent foramen ovale (PFO). The small opening between the upper chambers of the heart can increase the risk of recurrent ischemic stroke and transcatheter closure can avoid open heart surgery.
Abbott said the Talisman system improves the efficiency and effectiveness of the PFO occlusion procedure compared to the previous generation PFO occluder.
Expanding on the success of Abbott's Amplatzer PFO Occluder, the next-generation Talisman system offers an additional 30 mm device size, and all Talisman PFO occluders come pre-attached to the delivery cable, reducing preparation time for doctors. The FDA also cleared the Amplatzer Talisman Delivery Sheath, used to deliver the occluder during implantation.
A PFO is a hole in the heart when the foramen ovale – a flap-like opening that is a normal part of fetal development allowing oxygenated blood to pass through the baby's heart and bypass the lungs – does not close as it should following birth. About one in four people have a PFO, where the foramen ovale stays open, or is "patent." A PFO may cause stroke by allowing blood clots to pass from the right side of the heart to the left, from where they may travel to the brain. For patients who have suffered a stroke as a result of their PFO, physicians may opt for occlusion (or closure) of the PFO through a minimally invasive procedure using therapy like the Talisman system to seal off the opening to reduce the risk of another stroke.
Abbott's next-generation Talisman system builds off the company's industry-leading Amplatzer PFO Occluder, which has more than two decades of safety and effectiveness data and is the only approved solution in the U.S. designed specifically to close the PFO. The Talisman system provides the widest range of PFO occluder sizes in the U.S. through the addition of the new 30 mm offering. Additionally, the Talisman device comes pre-attached to the delivery cable, reducing prep time ahead of the procedure and making it easier to use. Talisman is fully recapturable and repositionable to ensure optimal placement.
"Extensive clinical trial data and the latest guidance from industry organizations support PFO closure as an important treatment option to reduce risk of recurrent stroke in patients," said Lee MacDonald, M.D., structural cardiologist at South Denver Cardiology Associates. "With the new Talisman system now available in the U.S., doctors can treat a broader range of patient anatomies, and the preparation needed for PFO occlusion procedures is simpler and faster."
For more information on the benefits of PFO closure, see guidance from the American Heart Association/American Stroke Association and the American Academy of Neurology.
For U.S. safety information on Talisman: https://abbo.tt/AmplatzerTalismanISI
Related PFO Closure Content:
VIDEO: PFO Closure Shows Continued Stroke Prevention Benefit at 5 Years — Interview with Scott E. Kasner, M.D.
VIDEO: How Transcatheter PFO Closure Can Reduce Cryptogenic Stroke — Interview with John Rhodes, M.D.
Gore Cardioform ASD Occluder Receives FDA Approval
VIDEO: Transcatheter PFO Closure to Prevent Stroke and Migraines — Interview with Carey Kimmelstiel, M.D.
SCAI Offers Recommendations for Safe Use of PFO Closure Technologies
VIDEO: An Overview of PFO Closure to Treat Cryptogenic Stroke — Interview with Karen Orjuela, M.D.
VIDEO: Demonstration of a Transcatheter PFO Occluder Implantation
VIDEO: Transcatheter Closure of Holes in the Heart — Interview with Ziyad Hijazi, M.D.
PFO Closure Shows Positive Results from REDUCE Clinical Study


 June 20, 2024
June 20, 2024