November 21, 2014 — Agfa HealthCare will showcase its updated Enterprise Imaging Xero Viewer at RSNA 2014. With its new ...
Diagnostic and Interventional Cardiology DAIC
Diagnostic and Interventional Cardiology (DAIC) is a magazine that reaches more than 25,000 healthcare professionals in cardiology, interventional cardiology and cath labs across the United States. These influential buying team members rely on DAIC's award-winning editorial content and comparison charts as a unique research tool for specifying, recommending and approving technology/device purchases.
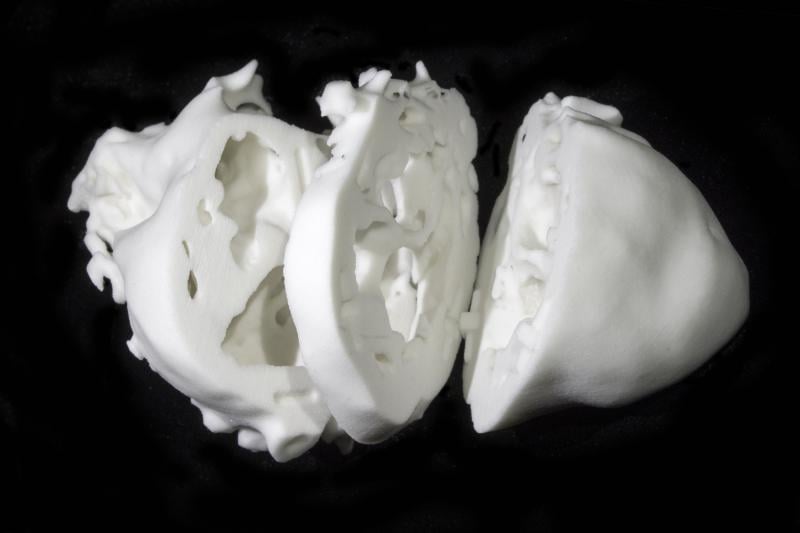
November 20, 2014 — An experimental 3-D printed model of the heart may help surgeons treat patients born with ...
November 20, 2014 — Oxford University researchers presented data that reveal the extent to which smoking causes silent ...
The new ResolutionMD functionality, which will be showcased at the 2014 RSNA conference by Calgary Scientific, offers ...
November 20, 2014 – The Centers for Medicare & Medicaid Services (CMS) released final rules outlining how Medicare will ...
November 19, 2014 — At the 2014 Radiological Society of North America (RSNA) meeting, Cerner Corp. will feature RadNet ...
November 19, 2014 — Carestream will debut its Carestream Touch Ultrasound System that offers a combination of enhanced ...
Routinely adding mitral valve repair to coronary artery bypass graft surgery for heart attack patients may not be ...
November 18, 2014 — Vital Images Inc. announced enhancements in image management to its VitreaView enterprise viewer wit ...
November 18, 2014 — CIRS will feature a variety of quality assurance (QA), research and training phantoms for all ...
November 18, 2014 — Radcal Corporation announced the release of its first WiFi device, the Nugget, which will be ...
November 18, 2014 — Pierre Galvagni Silveira, M.D., Ph.D., from the Federal University of Santa Catarina in ...
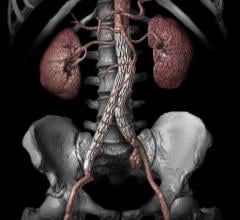
November 17, 2014 — VIVA Physicians, a not-for-profit organization in the field of vascular medicine and intervention ...
November 17, 2014 — Siemens Healthcare has introduced the Symbia Evo Excel SPECT (single-photon emission computed ...
November 14, 2014 — Boston Scientific Corp. announced that its subcutaneous implantable defibrillator (S-ICD System) ...


 November 21, 2014
November 21, 2014