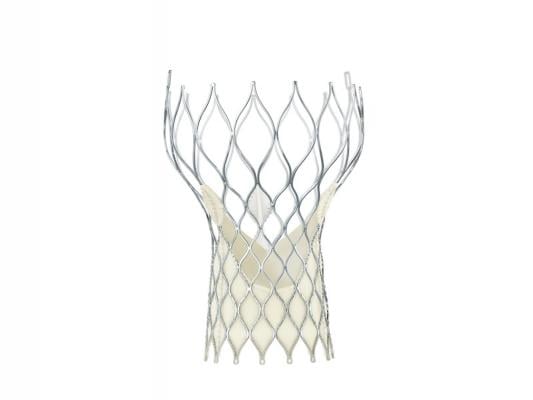
September 4, 2014 — Medtronic announced CE mark for the 23 mm CoreValve Evolut R system for transcatheter aortic valve implantation (TAVI). The novel self-expanding valve and 14 French equivalent delivery system offers new capabilities that advance valve performance and deliverability during the procedure, while providing the option to recapture and reposition the valve during deployment phase if needed.
“The CoreValve Evolut R system offers improvements to a proven TAVI technology platform,” said Eberhard Grube, M.D., head of the Center of Innovative Interventions in Cardiology (CIIC), University Hospital Bonn, Germany. “The system’s new recapture-enabled capabilities and advancements in valve delivery provide physicians with added procedural confidence. It’s a significant advance to know there is the option to redeploy the valve in the ideal position if necessary.”
The novel system, consisting of the CoreValve Evolut R transcatheter aortic valve and the EnVeo R delivery catheter system, is designed for first-time positioning accuracy and also offers a new InLine sheath that significantly reduces the profile to the lowest on the market (14 French equivalent, less than 1/5 inch), as a smaller profile size is believed to minimize the risk of major vascular complications. The new valve is anatomically designed to increase conformability at the annulus for optimal annular fit and sealing, while maintaining supra-annular valve position for improved hemodynamic performance.
“Built on the proven foundation and procedural success of the CoreValve system with more than 65,000 implants worldwide, the CoreValve Evolut R system is the future of transcatheter aortic valve replacement,” said Rhonda Robb, vice president and general manager, Heart-Valve Therapies, Medtronic. “CoreValve Evolut R provides heart teams with meaningful advancements that will increase the potential for optimal device placement.”
The 23 mm CoreValve Evolut R transcatheter valve and EnVeo R delivery catheter system are now available in Europe and other countries that recognize the CE mark. It is not approved for commercial use in the United States, where it is currently undergoing clinical trials.
For more information: www.medtronic.com


 December 24, 2025
December 24, 2025 









