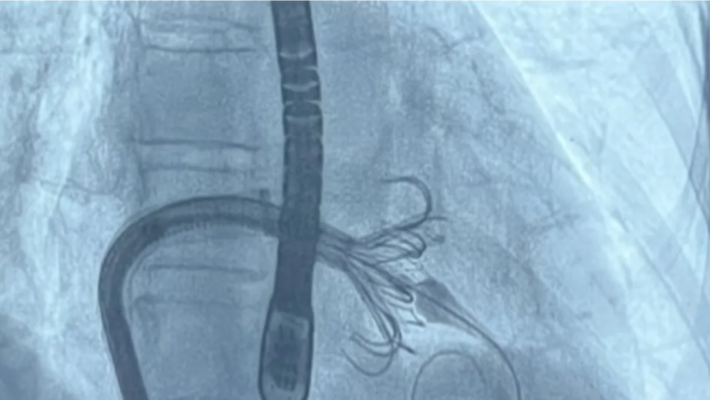
June 21, 2024 — UC San Francisco interventional cardiologists and interventional echocardiographers recently performed the health system’s first commercial transcatheter tricuspid valve replacement procedure using the Edwards EVOQUE system. The novel and minimally invasive cardiac procedure was recently approved for use within the U.S. by the Food and Drug Administration. The UCSF team is the first to perform the commercial procedure across the UC system and in San Francisco and is one of only a handful of centers currently performing the procedure nationally.
The EVOQUE tricuspid valve replacement system is a device designed to treat patients with severe leaking of the tricuspid valve, known as tricuspid regurgitation, without open heart surgery. The EVOQUE valve is implanted using a minimally invasive delivery system (a catheter) through the femoral vein reaching to the heart.
“Patients suffering with debilitating symptoms as a result of tricuspid regurgitation represent a large and significantly underserved patient group,” said Sammy Elmariah, MD, UCSF chief of Interventional Cardiology. “The EVOQUE tricuspid valve replacement system represents a major advance for a group of patients who previously had limited treatment options.”
Tricuspid regurgitation occurs when the tricuspid valve in the heart does not close completely, usually because the valve or surrounding structures have dilated or stretched, preventing the valve leaflets from closing tightly. Blood then flows backwards within the heart and may cause symptoms such as shortness of breath and swelling in the abdomen, legs or veins in the neck.
UCSF’s multidisciplinary heart valve team began performing the tricuspid valve replacement procedure using the Edwards Lifesciences EVOQUE system in 2023, as part of the TRISCEND II clinical trial evaluating the safety and effectiveness of the EVOQUE system.
“The recent FDA approval of the EVOQUE tricuspid valve replacement system is a monumental step forward in transcatheter options for the oft forgotten tricuspid valve,” said Neal Shah, MD, a UCSF cardiologist who specializes in cardiac imaging. “With a clear percutaneous option now available, we can provide advanced treatments to patients who would otherwise not receive definitive care for their leaky valve.”
In addition to minimally invasive tricuspid valve replacement, Elmariah and the interventional cardiology team are also investigating how to increase access to other heart valve disease treatments and improve patient involvement in the management of their own care.
For more information: https://ucsfhealth.org
Related Content:
Edwards Evoque Transcatheter Tricuspid Valve Replacement System Receives CE Mark
Edwards Announces One-year Data on Transfemoral Transcatheter Tricuspid Valve Replacement
Six-Month Outcomes Positive for Transfemoral Tricuspid Valve in Tricuspid Regurgitation Patients


 December 24, 2025
December 24, 2025 









