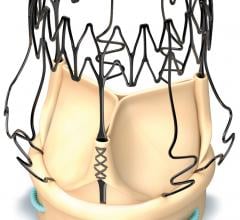
August 31, 2017 — NaviGate Cardiac Structures Inc. (NCSI) announced that its Gate catheter-guided tricuspid ...
Diagnostic and Interventional Cardiology DAIC
Diagnostic and Interventional Cardiology (DAIC) is a magazine that reaches more than 25,000 healthcare professionals in cardiology, interventional cardiology and cath labs across the United States. These influential buying team members rely on DAIC's award-winning editorial content and comparison charts as a unique research tool for specifying, recommending and approving technology/device purchases.
August 30, 2017 — Vascular Dynamics Inc. announced interim results of the company’s first-in-human trial of its MobiusHD ...
Azeem Latib, M.D., MBBCh, FCP, interventional cardiologist at Columbus Hospital in Milan, Italy, discusses the latest ...
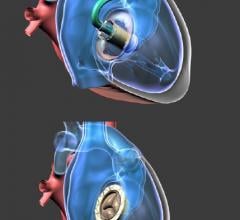
August 29, 2017 — Abbott announced it has received U.S. Food and Drug Administration (FDA) approval for its Full MagLev ...

August 29, 2017 — The U.S. Food and Drug Administration (FDA) approved a firmware update that is now available to reduce ...
This video educational session, provided in partnership with the American Society of Echocardiography (ASE), is titled ...
August 23, 2017 — Merit Medical Systems Inc. will partner with internationally renowned interventional cardiologist ...
August 23, 2017 — PinnacleHealth is the first hospital in Pennsylvania and one of the first 10 in the country to ...
August 22, 2017 — LivaNova PLC announced its Perceval sutureless aortic heart valve received approval from the Centers ...
August 21, 2017 — Biotronik announced U.S. Food and Drug Administration (FDA) approval and commercial availability of ...
The Centers for Medicare and Medicaid Services (CMS) in August announced a proposed rule to cancel the Episode Payment ...

August 17, 2017 — Cybersecurity has become a growing concern in healthcare as patient data, medical systems and ...
August 17, 2017 — Houston Methodist Hospital and Siemens Healthineers have entered into a multi-year agreement to bring ...
August 16, 2017 — BioCardia Inc. recently announced completion of treatment for the 10-patient roll-in cohort for the ...

August 16, 2017 — The Centers for Medicare and Medicaid Services (CMS) announced a proposed rule to reduce the number of ...

 August 31, 2017
August 31, 2017