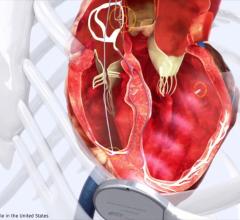
May 11, 2017 — Biotronik announced U.S. Food and Drug Administration (FDA) approval of the company's MultiPole Pacing (MPP) technology, providing physicians with additional treatment options for heart failure patients who have been non-responsive to cardiac resynchronization therapy (CRT). MPP will be available on new Biotronik CRT defibrillator (CRT-D) systems for patients with heart failure.
Nearly 40 percent of heart failure patients are initially non-responsive to CRT. Biotronik's MPP technology addresses this challenge by enabling the left ventricle to be paced twice per cardiac cycle. Uniquely, these paces can be either sequential or simultaneous, allowing for greater customization of therapy to meet specific patient needs. The company’s CRT-D systems include MPP and feature ProMRI technology, providing patients with access to critical diagnostic imaging scans as needed. These devices are also equipped with MRI AutoDetect, a dedicated sensor that detects the magnetic resonance imaging (MRI) environment, converts the patient's device to MRI mode, and then automatically returns to its permanent program when the scan is complete.
"MultiPole Pacing is an important technology that allows physicians to tailor cardiac resynchronization therapy to each patient," said Gery Tomassoni, M.D., Baptist Health, Lexington, Ky. "Heart failure is a complex condition and physicians are routinely challenged to find the ideal treatment for unique disease presentations. Adding MPP technology to other key Biotronik features creates more options for physicians to meet evolving patient needs."
Biotronik will feature MPP and other 360° CRT solutions at the 2017 Heart Rhythm Society (HRS) Annual Meeting, May 10-13 in Chicago.
For more information: www.biotronik.com


 July 21, 2025
July 21, 2025