June 26, 2020 — Abbott announced new data from the company's LightLab Initiative that showed optical coherence ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
June 26, 2020 – New data from the Global SYMPLICITY Registry (GSR) showed that renal denervation (RDN) with the ...
June 23, 2020 — The U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Program status for the ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
Prior to January 2020 when clinicians read about the history of the 1918 flu, and epidemiologists predicted we were ...
June 19, 2020 — SMT (Sahajanand Medical Technologies Pvt. Ltd) said it acquired of the structural heart medical device ...
June 19, 2020 — iVascular SLU announced the global launch of Essential Pro, a novel coronary artery drug-coated balloon ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
June 17, 2020 — MedAlliance announced its second CE mark approval for its Selution SLR 0.014 percutaneous transluminal ...
June 16, 2020 — The 36-month results from Veryan Medical’s MIMICS-2 study for the BioMimics 3D femoropopliteal stent ...
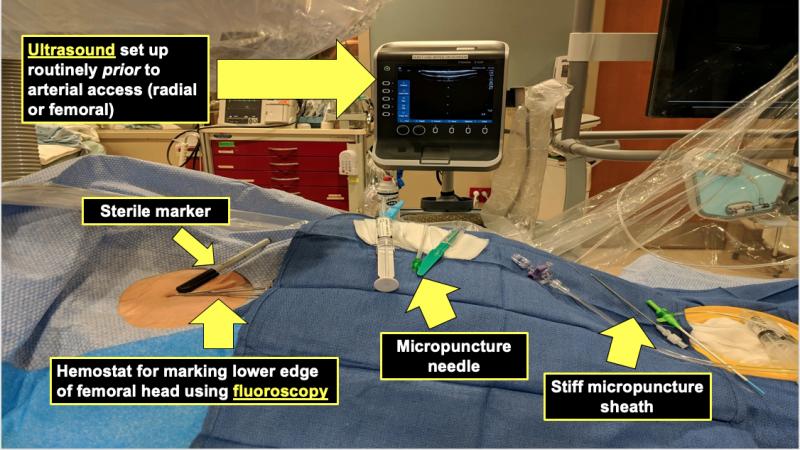
Vascular access site bleeding is associated with higher complications and mortality rates. For decades femoral access ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...

June 9, 2020 — SMT (Sahajanand Medical Technology Private Ltd.), a leading medical device company in India focused on ...
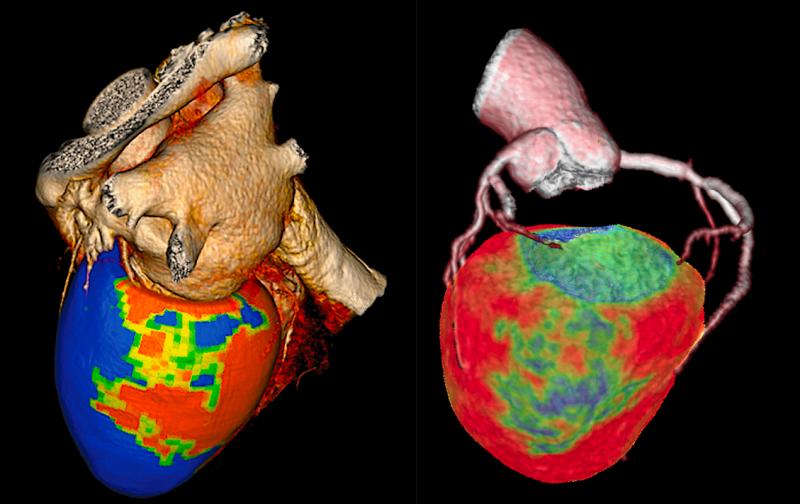
Here is an overview of a few of the biggest technology advances in cardiovascular computed tomography (CT). These are ...
June 8, 2020 — Edwards Lifesciences Corp. announced Chinese regulatory approval for the Edwards Sapien 3 transcatheter ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
June 8, 2020 – BD (Becton, Dickinson and Company) launched the Halo One Thin-Walled Guiding Sheath, designed to perform ...

It was originally thought novel coronavirus (COVID-19, SARS-CoV-2) was primarily a respiratory disorder, but as larger ...
June 5, 2020 — Abiomed announced the U.S. Food and Drug Administration (FDA) has approved the company's investigational ...


 June 26, 2020
June 26, 2020