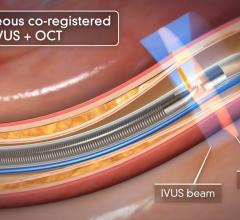
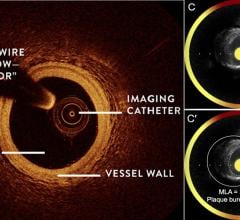
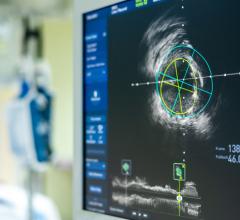
Optical Coherence Tomography (OCT)
Optical coherence tomography (OCT) is an catheter-based intravascular imaging technology that uses low-coherence light interferometry, using near-infrared light, to capture high-resolution 2-D and 3-D image reconstructions of coronary arteries. It has a lower level of tissue penetration than intravascular ultrasound (IVUS), but offers much higher resolution images of the tissues. It is used to determine the nature and extent of the atheroscleratic disease in the vessel wall, to assess in-stent restenosis and aid catheter procedure navigation in the cath lab.
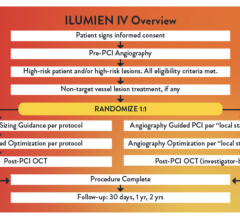
August 29, 2023 — Optical coherence tomography (OCT) is non-inferior to intravascular ultrasound (IVUS) for guiding ...
August 29, 2023 — Abbott announced late-breaking data from the first-of-its-kind ILUMIEN IV OPTIMAL PCI (ILUMIEN IV) ...
Conavi Medical Inc., a leader of hybrid imaging guidance for common minimally invasive heart procedures, plans to expand ...
December 15, 2021 — Conavi Medical Inc. has entered into agreements for over $20 million in funding to support ...
August 5, 2021 — The U.S. Food and Drug Administration (FDA) has cleared clearance Abbott's latest optical coherence ...
May 12, 2021 — Abbott recently announced its new interventional imaging platform powered by Ultreon 1.0 Software, has ...
February 16, 2021 – SpectraWave Inc. announced a $13.2 million series A-2 financing to support completion of product ...
November 17, 2020 — Diagnostic imaging techniques were able to find the underlying cause of heart attack in many women ...

Fractional flow reserve (FFR) is considered the gold standard measure whether a coronary lesions needs a percutaneous ...
October 18, 2020 – Data from the COMBINE (OCT-FFR) study found that the use of FFR combined with OCT imaging can help ...
October 1, 2020 — A team of researchers led by the University of Adelaide and University of Stuttgart has used 3-D micro ...

Although not a new technology, optical coherence tomography (OCT) is the dark horse of imaging that could give ...
September 14, 2020 — Avinger received U.S. Food and Drug Administration (FDA) 510(k) clearance from the FDA for its ...
Nick West, M.D., chief medical officer for Abbott, explains the details from a survey of 1,400 patients, physicians and ...
June 26, 2020 — Abbott announced new data from the company's LightLab Initiative that showed optical coherence ...

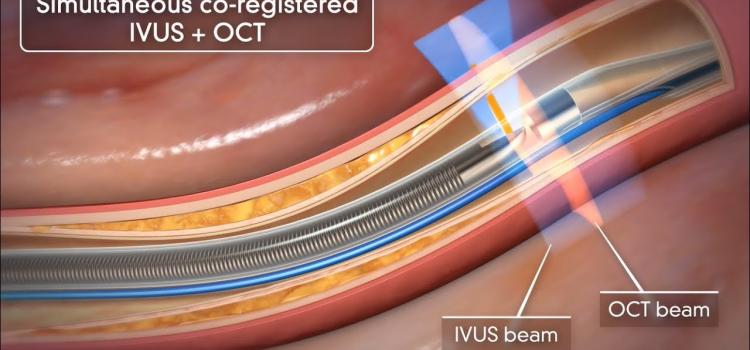



 August 29, 2023
August 29, 2023