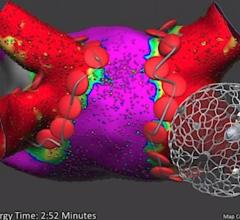
This is a quick animation demonstrating how the new 9 French Abiomed Impella ECP expands to approximately 18 French and ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
June 4, 2020 — Intra-operative transesophageal echocardiography (TEE) is a versatile diagnostic and monitoring tool used ...
June 4, 2020 — Transit Scientific announced the U.S. Food and Drug Administration (FDA) cleared the XO Score Percutaneou ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
June 4, 2020 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) magaz ...

The American College of Cardiology (ACC) together with other North American cardiovascular societies issued a framework ...

June 1, 2020 — The U.S. Food and Drug Administration (FDA) has issued an emergency use authorization (EUA) for the Abiom ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...

May 29, 2020 — As states begin to re-open as the number of COVID-19 patients declines, 40 percent of Americans still ...
Jay Mohan, D.O., RPVI, interventional cardiology fellow at William Beaumont Hospital, Royal Oak, Michigan, created this ...
May 29, 2020 — Neovasc Inc., developer of a minimally invasive transcatheter mitral valve replacement (TMVR) ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
May 28, 2020 — The rising incidence of cardiovascular disease has prompted people to seek timely treatment to improve ...
May 28, 2020 — As hospitals begin reopening to elective exams and procedures amid the COVID-19 pandemic, they need to be ...
May 28, 2020 – MedAlliance has announced the award of its second European CE mark clearance for its Selution SLR 0.014 ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
May 28, 2020 — Hoag Memorial Hospital Presbyterian recently became the first hospital on the West Coast to perform an ...
May 27, 2020 — To ensure the health and safety of all attendees due to the ongoing COVID-19 (SARS-CoV-2) pandemic, the C ...
Interview with Andrew D. Krahn, M.D., FHRS, head of the division of cardiology at St. Paul’s Hospital, and professor of ...


 June 05, 2020
June 05, 2020