July 3, 2007 - CryoCor, Inc. and Boston Scientific Corporation announced on June 28 a strategic collaboration in the ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
July 3, 2007 - Vascular Solutions Inc. announced that the FDA has granted 510(k) clearance for the Gopher support ...
June 29, 2007 - Investigators from the Wake Forest University (WFU) School of Medicine in Winston-Salem N.C. and ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
June 27, 2007 — REVA Medical, Inc. recently announced enrollment of the first patients in a first-in-man clinical trial ...
June 20, 2007 — Sono-Tek Corporation recently announced the release of the MediCoat PSI, a system which offers ...
June 20, 2007 — Sono-Tek Corporation recently announced the release of the MediCoat PSI.
The MediCoat PSI offers the ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
June 19, 2007 – Cardica Inc. and Cook Medical today announced that they have entered into an agreement to develop ...
June 18, 2007 — Kensey Nash Corporation today announced that it has completed a European Union (EU) study to ...
June 15, 2007 — Johnson & Johnson says U.S. regulators have ended almost three years of sanctions over manufacturing ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
June 13, 2007 - Ostial Solutions LLC announced that it has received FDA 510(k) clearance to market the Ostial Pro Stent ...
June 13, 2007 - Cordis Endovascular announced at the 61st Annual Meeting of the Society for Vascular Surgery the ...
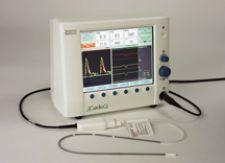
The company offers a monitor that uses a continuous wave doppler that can look right down into the aortic valve ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
June 4, 2007 — Biodegradable magnesium stents have been developed to treat blockages in coronary arteries. The ...
June 4, 2007 — Contrary to recent studies, proper use of a drug called aprotinin to reduce bleeding during heart ...
June 1, 2007 — Abbott announced it has completed its application to the U.S. Food and Drug Administration (FDA) ...

 July 02, 2007
July 02, 2007


