In a new, five-year agreement signed last week, Datascope’s InterVascular Division will become the distributor of Sorin Group’s peripheral vascular stent products worldwide, excluding the U.S. and Japan. Datascope will have an option to acquire that side of the Sorin business.
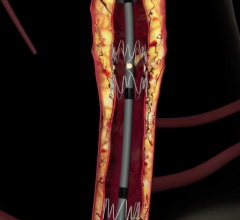
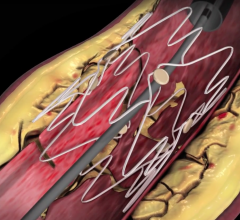
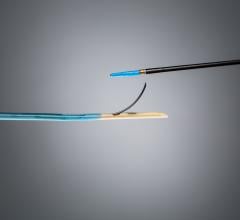
Sorin's family of peripheral stent products are dedicated to the treatment of peripheral arterial disease ("PAD") and use the Carbofilm Technology, comprising clinically proven biocompatible and hemocompatible characteristics. The product line includes balloon-expandable and self-expanding stent systems principally for use in the treatment of the iliac arteries, renal arteries and infrapopliteal lesions, as well as balloon systems for use in PTA (Percutaneous Transluminal Angioplasty).
Outside of the U.S. and Japan the global market for peripheral vascular stents and PTA balloons is estimated at $192 million annually, and growing due to an increase in diagnostic procedures as well as the aging population and higher incidences of diabetes, high blood pressure, obesity and hypercholesterolemia -- all major risk factors for PAD.
For more information visit www.datascope.com.


 November 06, 2019
November 06, 2019