September 17, 2009 – NMT Medical Inc. said yesterday it will commence data analysis for its landmark STARFlex ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
September 17, 2009 – Boston Scientific Corp. announced its schedule of the company's major events and press ...
September 17, 2009 – Elixir Medical Corp. said today its stent systems using bioabsorbable polymers will be ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...

In August Daiichi Sankyo and Eli Lilly and Company launched U.S. sales of Effient (prasugrel), a new antiplatelet ...

Ruptured vulnerable plaque (VP) is blamed for the majority of acute myocardial infarctions and many strokes, but ...
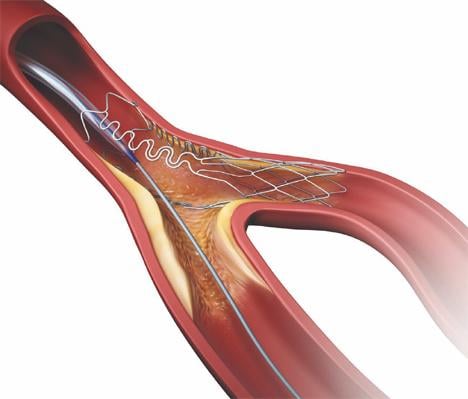
Stenting of lesions at coronary vessel bifurcations is one of the most challenging percutaneous interventions ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...

Over the past year several new drug-eluting stents (DES) stents have been released and SYNTAX trial data proved ...

Metal stents are foreign objects that can irritate vessels and cause stent thrombosis, prevent vessel ...

The polymer coating on drug-eluting stents (DES) used to carry the antiproliferative drugs cause an increased risk ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
September 16, 2009 – Texas Cardiac Arrhythmia Institute at St. David’s Medical Center in Austin, Texas plans to ...
September 16, 2009 – Hansen Medical Inc. today said it received FDA clearance for its next generation Sensei X ...
September 15, 2009 – Tryton Medical Inc. said today its Tryton Side Branch Stent has been used in 250 procedures ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
September 14, 2009 – OrbusNeich's Genous Bio-engineered R stent is feasible and safe in patients who need ...
Sept. 14, 2009 – Abbott said today the Chinese State Food and Drug Administration (SFDA) has approved the XIENCE ...
September 14, 2009 – InfraReDx said it will highlight its LipiScan Coronary Imaging System at the Cardiovascular ...


 September 17, 2009
September 17, 2009









