Recently, concerns have been raised that transradial (TR) cardiac catheterization significantly increases radiation ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).

A major issue with clopidogrel is that one-third of patients are nonresponders because they lack the enzymes to ...

The standard-of-care for dual antiplatelet therapy (DAPT) for more than a decade has been clopidogrel (Plavix) plus ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
August 23, 2012 — Decision Resources, a research and advisory firm for pharmaceutical and healthcare issues, finds that ...
August 23, 2012 – Philips Healthcare and Corindus Vascular Robotics announced a distribution agreement for Corindus’ ...
August 22, 2012 — Placement of an endovascular stent has become a common procedure for successfully reopening blocked ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...

August 22, 2012 — Comedian Rosie O'Donnell’s heart attack last week is helping increase public education about how women ...
August 20, 2012 — Abbott today announced that the Xience Xpedition everolimus-eluting coronary stent system received CE ...
August 15, 2012 — Sound Interventions Inc. has released three-month data from the company's first-in-human clinical ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
August 15, 2012 — Previously unreleased three-year data from the Zilver PTX randomized controlled trial of paclitaxel ...
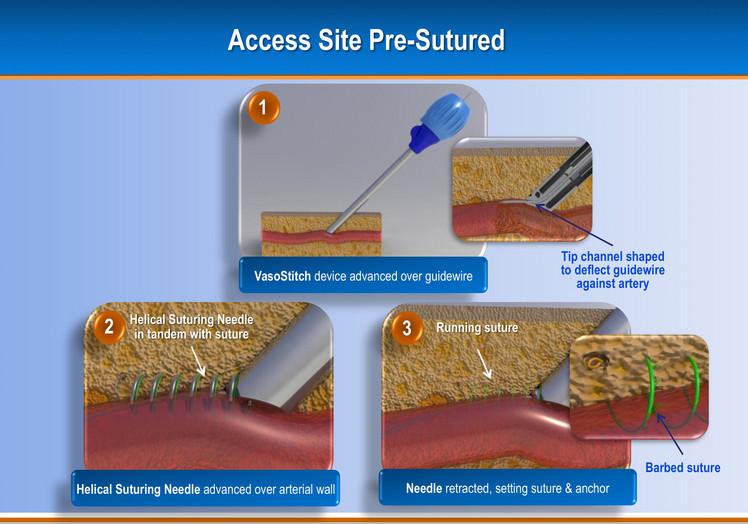
Due to the increasing number of transcatheter aortic valve replacements (TAVR) and endovascular aneurysm repair (EVAR) ...
August 14, 2012 — The next five years will see many hospitals in Europe overcoming the high cost and complexity of ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
August 14, 2012 — The U.S. Food and Drug Administration (FDA) has granted 510(k) market clearance to Stryker ...
August 14, 2012 — The U.S. Food and Drug Administration (FDA) is hosting a public workshop that examines use of ...

August 13, 2012 — Providers are increasingly focused on using interventional labs in a hybrid operating room (OR) enviro ...

 August 28, 2012
August 28, 2012









