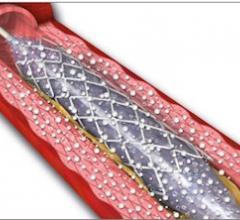
September 26, 2012 — Abbott Vascular this week began its international launch of the Absorb coronary stent, the world's ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).

September 25, 2012 — According to Millennium Research Group (MRG), the global authority on medical technology market ...
September 24, 2012—The new U.S. Food and Drug Administration Safety and Innovation Act (FDASIA) imposes many Medical ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
September 24, 2012 — Bio DG announced that its patent for a novel metal system, to use as biodegradable material in ...
September 21, 2012 — Following the success of Biotronik’s Pulsar-18 0.018-inch, 4 French platform self-expanding stent ...
September 21, 2012 — Cardionovum GmbH announced that the results of a preclinical study and a first-in-man clinical ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
September 20, 2012 — Boston Scientific Corp. has signed a definitive agreement to acquire BridgePoint Medical Inc., a ...
September 19, 2012 — Heart catheter procedures guided by magnetic resonance imaging (MRI) are as safe as X-ray angiograp ...
September 18, 2012 — Inpatient hospital treatment accounts for the largest proportion of healthcare spending in the ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
September 18, 2012 — Neovasc Inc. announced that acute results from preclinical studies of its Tiara valve for the ...
September 18, 2012—Covidien announced the launch of the Viance Crossing Catheter and Enteer Re-entry System for treating ...
September 17, 2012 — The American College of Cardiology (ACC) has released the results of its 2012 Practice Census ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...

Cardiologists are restricting the use of transcatheter aortic valve implantation (TAVI) to very old or very sick ...
September 17, 2012 — The Cardiovascular Research Foundation (CRF) announced the late-breaking trials and first report ...
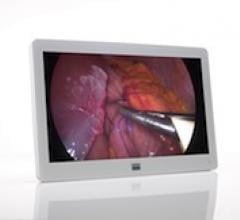
September 14, 2012 — Barco announced the launch of its MDSC-2226, a 26-inch surgical display providing full ...


 September 26, 2012
September 26, 2012