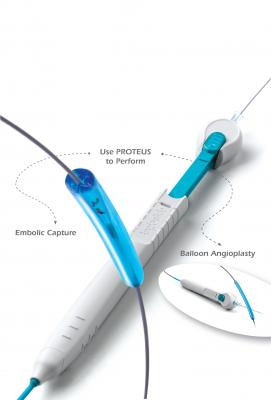
May 4, 2012 - Angioslide Ltd., a provider of embolic capture angioplasty solutions, announced that it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its new 3x100 mm Proteus device for treating peripheral artery disease in below-the-knee (BTK) vasculature. The new 3/100 mm device accommodates 0.014-inch guidewires, which significantly broaden the potential use of Proteus technology.
Angioslide’s proprietary technology combines the functionality of a balloon angioplasty device with the addition of built-in embolic capture. Proteus is a competitive percutaneous transluminal angioplasty (PTA) balloon with features similar to leading PTA balloons (deflation time, pushability, crossing profile). Then, during deflation, the Proteus captures and retrieves potentially harmful embolic material. The capture of embolic material is enabled by the inward folding of the balloon, which creates a low-pressure cavity. The negative pressure in this cavity generates an aspiration effect, pulling embolic material into the cavity. When retrieved through the sheath, Proteus removes the captured material from the body.
BTK interventions are often characterized by patients with long and diffuse lesions, diabetic foot ulcers and/or critical limbs ischemia. Proteus 3x100 offers physicians, for the first time, the ability to capture and remove embolic material with a single device during these challenging interventions.
“Introduction of the new dedicated, BTK, low-profile 0.014-inch Proteus platform supports Angioslide's continuing commitment to improve procedure outcomes. Distal embolic complications in this compromised group of patients further risk blood flow to the foot. Proteus will become a necessary device in the 'tool-box' of physicians practicing "endovascular-first" approach,” said Lihu Avitov, Angioslide CEO.
For more information: www.angioslide.com


 June 13, 2024
June 13, 2024 









