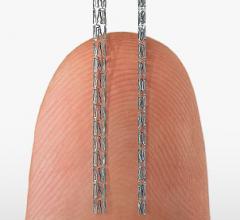
September 26, 2014 — The first large prospective study to examine the effectiveness of laser atherectomy in the ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
September 25, 2014 — Svelte Medical Systems reported that its drug-eluting coronary stent Integrated Delivery System ...
September 24, 2014 — A new study that compares the use of an everolimus-eluting bioresorbable vascular scaffold (BVS) ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...

September 24, 2014 — Abbott announced positive one-year clinical results from ABSORB II, the world's first prospective ...
DAIC Editor, Dave Fornell, interviews Jim Hermiller, M.D., FACC, director of interventional cardiology, St. Vincent ...
John Stevens, chairman and CEO of HeartFlow, explains his company's computed tomography (CT)-based fractional flow ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
September 23, 2014 — New analyses and extended follow-up from the BRIGHT study demonstrated that bivalirudin remained ...

September 22, 2014 — A first-of-its kind study found that using a cerebral protection device during transcatheter aortic ...
September 22, 2014 — According to a new study, while it is common for patients who have migraine to have a patent ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
September 22, 2014 — According to a new study, patients receiving six months of dual antiplatelet therapy (DAPT) after ...

September 19, 2014 — According to a new study, transcatheter aortic valve replacement (TAVR) provided meaningful ...

September 19, 2014 — The largest prospective longitudinal study of acute myocardial infarction (MI) patients offers ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...

September 19, 2014 — A new study investigating different durations of triple therapy for anticoagulation after drug ...
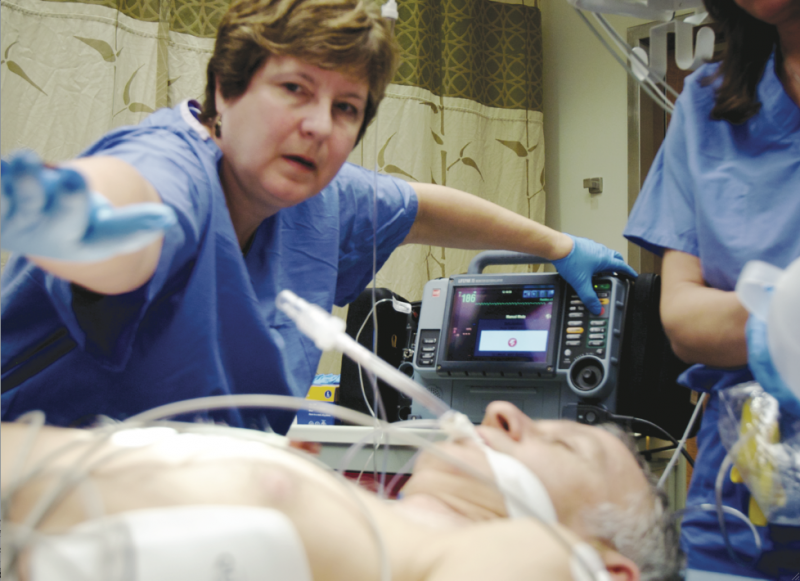
September 19, 2014 — According to a new study, peritoneal hypothermia is feasible for patients who have suffered ST ...
September 18, 2014 — New data from a landmark clinical trial found that after five years, transcatheter aortic valve ...

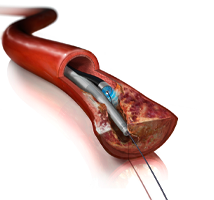
 September 26, 2014
September 26, 2014