While transcatheter aortic valve replacement (TAVR) is a paradigm shift in how valve disease is treated, one nagging ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
October 2, 2014 — ReFlow Medical Inc. announced the initial clinical use of its Wingman35 Crossing Catheter and speX ...
October 1, 2014 — St. Johns Hospital and Medical Center in Detroit recently became the first U.S. healthcare facility to ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
September 30, 2014 — Acist Medical Systems Inc., a Bracco Group company, showcased the HDi high-definition intravascular ...

Earlier this year, the Barnes-Jewish Hospital at Washington University School of Medicine in St. Louis adopted the ...

September 29, 2014 — Before undergoing cardiac imaging procedures involving radiation, healthcare providers should help ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
September 26, 2014 — In response to new science showing that complete revascularization of all significantly blocked ...
By Dave Fornell, editor of DAIC Magazine
The key take away messages from the 26th annual Transcatheter Cardiovascular ...
Doug Drachman, M.D., Mass General Hospital Institute of Heart, Vascular and Stroke Care, explains how to prevent and ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
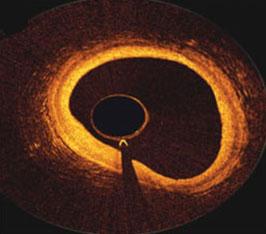
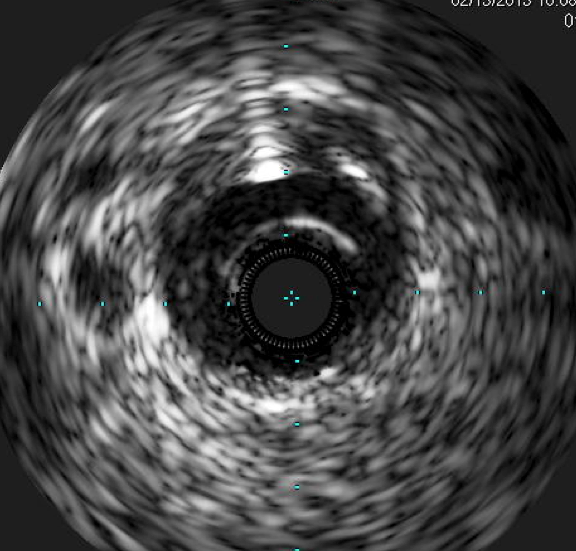

During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
Ziad Ali, M.D., senior scientist at the Cardiovascular Research Foundation (CRF), discusses the current trials and ...
DAIC Editor Dave Fornell offers his choices of the most innovative new cardiovascular technologies discussed in sessions ...


 October 02, 2014
October 02, 2014








