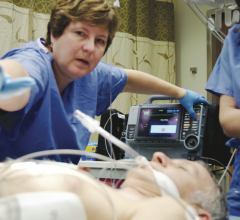
December 16, 2014 — A faster, coordinated emergency response in collaboration with hospital cardiac catheterization ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
December 15, 2014 — As the first integration between Interventional Radiology (IR) and CT technology, Toshiba’s Infinix ...
December 12, 2014 — Siemens Healthcare presented two new clinical applications for angiography at the 2014 Radiological ...
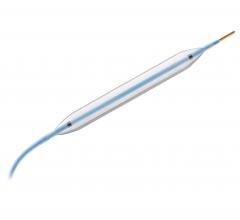
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
December 11, 2014 — Researchers at Okayama University in collaboration with several medical centers in Japan have ...
December 10, 2014 — In the first successful United States trial of a bioabsorbable polymer stent, the Boston Scientific ...
December 9, 2014 — CardioKinetix Inc. announced it has completed a $50 million financing led by Edwards Lifesciences ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...

December 9, 2014 — A study published in the Journal of the American College of Cardiology (JACC) shows intravascular ...
December 4, 2014 — Covidien announced that it received U.S. Food and Drug Administration 510(k) clearance for its Fortre ...
December 2, 2014 – Results of a new study challenge the current consensus in cardiology that peak myocardial edema only ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
December 4, 2014 — Biodex showcased its latest line of medical imaging tables and Clear-Lead Shielding at RSNA 2014.
...
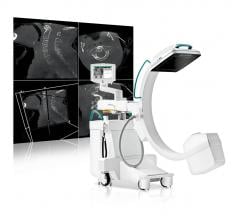
November 30, 2014 — At this year’s RSNA annual meeting, Ziehm Imaging will highlight Ziehm Vision RFD 3-D, a 3-D C-arm ...
November 26, 2014 — CompView Medical (CVM) debuts the M2u and M4u, its latest design of NuBOOM, an all-in-one equipment ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
November 21, 2014 — A coordinated emergency response by healthcare teams to treat heart attack patients meant faster ...



 December 16, 2014
December 16, 2014