February 19, 2016 — Nipro North America announced the launch of the AquaLiner II second-generation 0.035-inch ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
February 17, 2016 — Biotronik announced a partnership with Maquet Medical Systems USA to distribute Biotronik peripheral ...
February 17, 2016 — Up to 10 percent of people with high blood pressure have resistant hypertension — high blood ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
February 11, 2016 — Researchers funded by the National Institute of Biomedical Imaging and Bioengineering (NIBIB) used u ...

After five years of almost constant lobbying efforts and numerous attempts by the U.S. House to push through a repeal of ...
February 10, 2016 — CeloNova BioSciences Inc. announced this week that the first patient has been enrolled in its COBRA ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
The following are the late-breaking clinical trial presentations presented at the 2016 American College of Cardiology ...
February 9, 2016 — Boston Scientific Corp. announced the Centers for Medicare and Medicaid Services (CMS) will cover ...
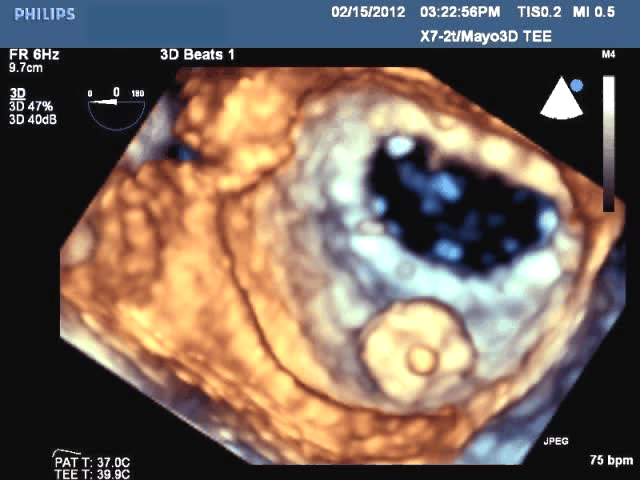
Off-label use of the St. Jude Amplatzer vascular plug devices offers a new solution for the minimally invasive repair of ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
February 8, 2016 — St. Jude Medical Inc. announced the launch of the company’s Optis Mobile System in Japan and Europe ...
February 8, 2016 — Stentys announced the first distribution agreements for its drug-eluting stent for treating below-the ...
February 4, 2016 — Stereotaxis and Philips have signed an addendum pursuant to their existing Development and ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
Medtronic announced Feb. 3 that the U.S. Food and Drug Administration (FDA) revised the CoreValve’s instructions for use ...
February 2, 2016 — Medtronic plc announced the recent CE Mark and commercial launch for an expanded size matrix of the ...
January 29, 2016 — Intact Vascular Inc. announced that positive six-month results from its Tack Optimized Balloon ...

 February 19, 2016
February 19, 2016