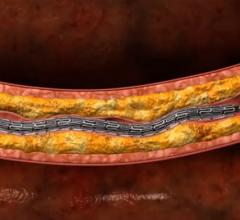
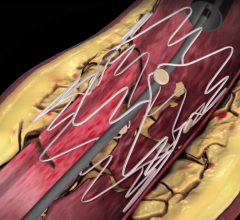
Millions of percutaneous coronary interventions (PCIs) are performed each year around the world to treat obstructive ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).


Acute pulmonary embolism (PE) can pose a major treatment quandary for clinicians. While massive (high-risk) PE is ...
Emory St. Joseph’s Hospital in Atlanta took control of more than $2.5 million in cardiovascular inventory by automating ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
The need for improved efficiency in healthcare and the new requirements of U.S. healthcare reform have rapidly made the ...
March 24, 2016 — Female cardiologists are less likely than their male counterparts to get married and have children ...
March 24, 2016 — Medinol announced the enrollment of their first patient in the U.S. NIRTRAKS Study. NIRTRAKS is a post ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
March 22, 2016 — PinnacleHealth CardioVascular Institute enrolled the first patient in Pennsylvania into the TOBA II ...

DAIC readers chose the following stories as the most popular content in 2015, based on website analytics. The list is ...
March 21, 2016 — The use of novel oral anticoagulants (NOACs) will see a substantial rise after an initially sluggish ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
March 17, 2016 — Boston Scientific announced in late February that the Eluvia Drug-Eluting Vascular Stent System ...
While there are constant advances in cardiac technologies, it is rare when one of these new ideas may rapidly challenge ...

March 15, 2016 — An independent panel of experts convened by the U.S. Food and Drug Administration (FDA) voted 9 to 0 ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
March 14, 2016 — Siemens Healthcare announced the U.S. Food and Drug Administration (FDA) has cleared three brand-new ...
March 11, 2016 — Magnesium Elektron, developer, manufacturer and supplier of high-performance magnesium alloys ...
March 11, 2016 — Teleflex Inc. announced the worldwide recall of Arrow International intra-aortic balloon catheter kits ...

 March 28, 2016
March 28, 2016