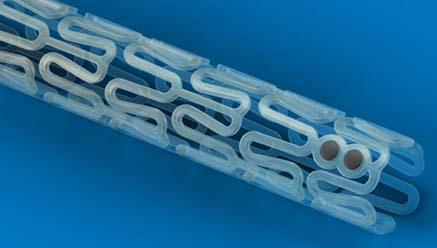
January 10, 2011 – European CE mark approval was announced today for the Abbott, Absorb drug-eluting bioresorbable coronary stent. It is the first bioresorbable stent to gain regulatory approval anywhere in the world. Full commercial launch in Europe is planned by the end of 2012.
January 7, 2011 – A new study from The Ohio State University found that Protandim can suppress intimal hyperplasia, the over-proliferation of cells that line the walls of blood vessels. The adverse blood vessel blockage often limits the effectiveness of several types of vascular surgery.
January 7, 2011 – The United States Patent and Trademark Office (USPTO) has granted an additional patent expanding protection of endothelial progenitor cell (EPC) capture technology. It applies to OrbusNeich’s Genous EPC technology.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
January 7, 2011 – A new access device is now available in cath labs across the United States. The Arstasis One Access Device, from Arstasis, is the first commercially available alternative to the Seldinger Technique in 52 years. Previously, only a select number of physicians participating in Arstasis clinical trials and select pre-launch registries have been able to use the device.
January 6, 2011 – The Heart Rhythm Society (HRS) recently hosted its inaugural two-day research forum to identify opportunities and challenges to advancing research in electrophysiology (EP).
January 6, 2011 – Boston Scientific has completed its acquisition of transcatheter valve developer Sadra Medical.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
January 6, 2011 - Healthcare spending in the United States grew 4 percent in 2009, to $2.5 trillion, or $8,086 per person, the slowest rate of growth in the 50-year history of the National Health Expenditure Accounts (NHEA). This was due in great part to the economic recession.
December 5, 2010 – Additional lots lots of the AngioSculpt Percutaneous Transluminal Angioplasty (PTA) Scoring Balloon Catheters are being recalled by the AngioScore Inc. The class I recall was originally initiated in December 2009, but the FDA reissued the alert in September.
January 5, 2011 — The U.S. Food and Drug Administration (FDA) has given clearance for Medic Vision Ltd. to market its SafeCT product in the United States. SafeCT processes and enhances images spanning a broad range of clinical applications acquired on a wide variety of computed tomography (CT) scanners.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
January 4, 2011 - The Centers for Medicare and Medicaid Services (CMS) and the Office of the National Coordinator for Health Information Technology (ONC) yesterday announced the availability of registration for the Medicare and Medicaid electronic health record (EHR) reimbursement incentive programs.
January 4, 2010 – The U.S. Food and Drug Administration (FDA) has released an update of what medical devices it expects to be developed and brought to market within the next 10 years.
January 4, 2011 - The Office of the National Coordinator for Health Information Technology (ONC) has issued a final rule to establish the permanent certification program for health information technology.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
January 4, 2011 – An automated, digital blood pressure device is now available in the United States and Canada. The Connex ProBP 3400, from Welch Allyn, fits in the palm of the hand and provides a portable option for capturing reliable blood pressure readings in virtually any hospital or primary care environment.
January 3, 2011 — A portable viewing application that interfaces with an existing RIS/PACS/cardiology PACS for access to images and reports has been introduced.
January 3, 2011 – Two new classes — “Advanced Computed Tomography (CT) Imaging for Technologists” and “Advanced Non-Contrast Magnetic Resonance Angiography (MRA)” — are being offered to educate technologists on the latest applications available with their Toshiba technology. They are offered by Toshiba America Medical Systems Inc.’s Education Center in Irvine, Calif.

 January 10, 2011
January 10, 2011











