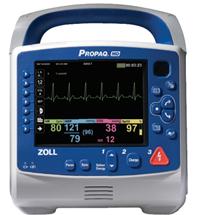
August 2, 2010 – The new Propaq MD Monitor/Defibrillator received 510(k) clearance from the U.S. Food and Drug Administration (FDA) and is specifically designed to meet the special needs of the military and air medical operations.
Development of this product was a joint undertaking between the Department of Defense and a cooperative arrangement between Welch Allyn and Zoll Medical Corp. Development was facilitated with grants from the U.S. Army Medical Research and Development Command.
The Propaq MD is an ultra-lightweight, compact device with highly sophisticated, advanced capabilities that combine the proven features of the Welch Allyn Propaq monitors with the therapeutic capabilities of Zoll defibrillation and noninvasive pacing technologies. The Propaq MD is 60 percent smaller and 40 percent lighter than other similar monitor/defibrillators. It is two pounds lighter than the current military vital signs monitor, the Propaq 206, even with defibrillation and pacing added.
The device provides a combination of capabilities that include a large, high-contrast color LCD display capable of viewing up to four waveforms simultaneously, as well as a full 12-lead electrocardiogram (ECG) for on-screen review. It also offers a unique night vision goggle (NVG) mode for military and air medical nighttime operation. All physiological monitoring parameter values, including heart rate, SpO2, ETCO2, respiration, noninvasive blood pressure, two temperatures, and three invasive pressures, are shown in large color-coded numeric formats. The device is capable of monitoring all patients, whether adult, pediatric or neonatal. Alarms are provided for all parameters. The Propaq MD is the only FDA-cleared airworthy defibrillator to provide monitoring of three invasive pressures necessary for treating critical patients during long transports.
A new battery system and AC power charger provides worldwide land, sea and air operating capability. The system can monitor all physiological parameters, including three invasive pressures and two temperature channels, for more than six hours on a single battery charge.
Trending Data
In addition to real-time monitoring, the Propaq MD has a full data collection and trending capability. Keys are provided to annotate all ACLS interventions such as intubation, drug administration, and associated ECG and other physiological waveform segments. Full trending data is available on the unit’s display from its integral memory, supporting data collection over 24-hour periods. Trends can include 1,000 time-stamped events and 32 snapshots of treatment. All data can be downloaded to a standard USB flash drive and transferred to electronic medical records using an open architecture XML format. An integrated printer can provide immediate documentation of events and diagnostic ECG traces.
Durability
Designed specifically for the tough demands of battlefield medicine, encompassing air transport and evacuation, and highly mobile ground, sea and air deployments of medical assets, the Propaq MD meets a number of military and international standards related to durability, environmental operation and storage extremes, radio frequency emissions, and susceptibility to spurious electrical and radio frequency noise. The Propaq MD is also rated at the most stringent IP55 rating for dust and water ingress.
In addition to the Propaq MD, a companion Propaq M model, which will be a monitor only version of Propaq MD, has been developed to have a completely identical and common interface for operation, batteries, power supplies, blood pressure cuffs, accessories, data and cables, but without therapeutic defibrillation and pacing capability. The Propaq M has not been reviewed and cleared by the FDA. These two products, when combined, will meet the most expansive needs of the military for patient care in austere environments. In addition to the common interfaces between the two units, virtually all existing Propaq monitor accessories, cables connectors, and Zoll M Series CCT defibrillation accessories currently used in military applications will be forward compatible with the new products.
For more information: www.zoll.com


 January 13, 2026
January 13, 2026 









