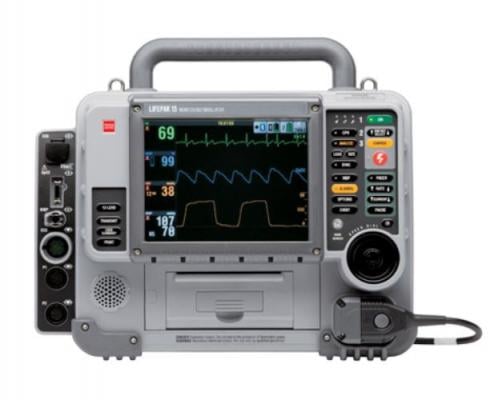
tryker is launching a voluntary field action on specific units of the LifePAK 15 defibrillator/monitors. The vendor said an issue has been identified where the devices to fail to deliver a defibrillation shock after the “Shock” button on the keypad is pressed.
January 13, 2020 — Stryker announced it is launching a voluntary field action on specific units of the LifePAK 15 defibrillator/monitors. The vendor said an issue has been identified where the devices fail to deliver a defibrillation shock after the “Shock” button on the keypad is pressed. This is a result of oxidation that may have formed over time within the button.
The company is contacting customers with impacted devices to schedule the correction of their device(s), which will include replacement of the affected keypad. Stryker anticipates that all devices subject to this field action will be serviced by June 2021. The company began contacting customers about the issue Dec. 27, 2019. EImpacted customers will be notified by letter and will be requested to verify their device status, Stryker said.
Most complaints associated with this issue were detected prior to patient use. Routine testing of the device can detect this fault condition, the company said.
The company is instructing customers to continue to use their LifePAK 15 monitor/defibrillator according to the operating instructions until the correction can be completed. Customers should continue to perform the daily check as described in the operator’s checklist, specifically, the Quik-Combo therapy cable check as described in the general maintenance and testing section (pages 10-4 and the LifePAK 15 monitor/defibrillator operator’s checklist, number 7).
If a customer experiences this issue, they should contact Stryker as soon as possible at 1-800-787-9537 and select option 2.
Customers with questions regarding this notification, please contact Stryker by calling 1-800-787-9537, option 2, 8 a.m. to 7 p.m. (EST), Monday – Friday, or by e-mail to MedTechSup@stryker.com or 1-800- 329-7879.
In addition to contacting Stryker, any potential quality problems or adverse reactions or events associated with the use of a product from Stryker may be reported online to the U. S. Food and Drug Administration’s MedWatch Safety Information and Adverse Event Reporting Program at www.fda.gov/safety/medwatch, by phone 1-800-332-1088.
Read the FDA recall notice: www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/stryker-launches-voluntary-field-action-specific-units-lifepakr-15-monitordefibrillator
Link to the Stryker product notice: www.strykeremergencycare.com/productnotices
The company issued a previous recall for the LiefPAK 15 in February 2019 for a different issue. Read the article "Stryker Warns LifePak15 Defibrillators May Lock Up After Shock is Delivered."


 August 15, 2024
August 15, 2024 








