December 13, 2010 – Based on emerging safety information, the pulmonary arterial hypertension (PAH) drug sitaxentan is being voluntarily withdrawn from the market. Pfizer is also discontinuing studies involving sitaxentan (Thelin) worldwide,
December 13, 2010 - One possible explanation for disparities in care for acute coronary syndrome between men and women may be that women are less likely to accept their physician’s recommendation on treatment. The results of the study were reported online today in Annals of Emergency Medicine.

Aortic stenosis (AS) is one of the most common valvular disorders in older adults, with a prevalence of approximately 8 percent at age 85 (1).
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...

There have only been incremental improvements in intravascular ultrasound (IVUS) over the past decade. However, three new technologies introduced in 2010, and a few more expected to enter trials or be commercialized in 2011, are pushing new frontiers. Characterizing Plaque

During a time when the U.S. healthcare reform is on the minds of many within the medical community, physicians and hospital administrators are continuously looking for ways to cut spending. One avenue that physicians and hospitals are exploring is to limit the use of expensive medical devices, such as drug-eluting stents (DES).

In the same fashion that the echocardiogram helped transform the cardiac exam, recent developments in the field of portable ultrasound are providing cardiologists with a new way to visualize the heart at the initial patient screening.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...

In a world where everything is unplugged and cordless is king, cardiology is constantly searching for and developing the technology to make patient care both better and more convenient.

For patients with severe hypertension that remains uncontrolled despite medical therapy, radiofrequency (RF) energy can be used in the renal vessels to block nerves involved with the sympathetic nervous system. Researchers believe this has the potential to impact the mechanical and hormonal activities of the kidneys and may eliminate the need for medical therapy.

Over the past few years, there have been three key areas of advancement in cardiovascular ultrasound technology – miniaturization, 3-D/4-D imaging and quantification measurements.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
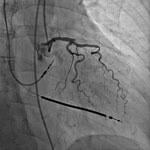
When it comes to interventional cardiology, increasing diagnostic confidence and reducing exam time are paramount to improving overall patient care.
December 10, 2010 – A new set of software applications that improves the quality of patient data and increases clinician productivity and efficiency has been launched. SentrySuite, by CareFusion, is designed for pulmonary and cardiopulmonary diagnostic settings. The software focuses on data, organization, workflow and connectivity so physicians can focus more time on patient care.
December 10, 2010 – An ECG management system is now offered with an IT solution for cardiac imaging. Siemens Healthcare now offers the ScImage PicomECG with its syngo Dynamics system.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
December 10, 2010 – The 5th annual list of the Top 10 Health Technology Hazards for 2011 is now available. The list, from the ECRI Institute, features the top health technology hazards that warrant critical attention by hospitals and other healthcare organizations.
December 10, 2010 – European CE mark approval has been granted so the Medtronic CoreValve transcatheter aortic valve replacement system can be delivered through the subclavian artery, located beneath the collar bone.
December 10, 2010 – The first commercial production of generators using molybdenum-99 (Mo-99) produced with low-enriched uranium (LEU) targets in the United States is underway. The Lantheus TechneLite (Technetium Tc99m) generator received the first commercial scale batch from NTP Radioisotopes, a subsidiary of the Nuclear Energy Corporate of South Africa (NESCA)

 December 13, 2010
December 13, 2010







