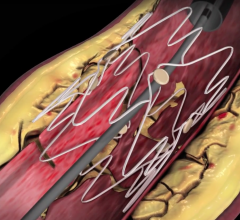
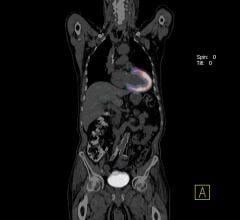
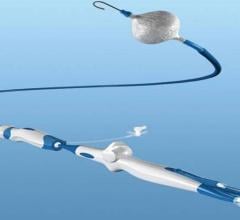
July 27, 2017 — There is good news when it comes to the heart’s sinoatrial node (SAN), the body’s natural pacemaker ...
The Spectranetics Corp. announced receipt of U.S. Food and Drug Administration (FDA) pre-market approval (PMA) of the Stellarex drug-coated balloon (DCB). The device is designed to restore and maintain blood flow to the superficial femoral and popliteal arteries in patients with peripheral arterial disease (PAD).
Intact Vascular Inc. recently announced that its Tack Optimized Balloon Angioplasty II Below the Knee (TOBA II BTK) clinical trial has commenced enrollment in Europe. The first patient was treated by Professor Dr. Marianne Brodmann and Dr. Peter Reif at Medical University Graz, Austria.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Siemens Healthineers has entered into a definitive agreement to acquire Epocal Inc., a subsidiary of Alere Inc. Epocal Inc. develops and provides point-of-care blood diagnostic systems for healthcare enterprises, including the epoc Blood Analysis System, a handheld, wireless testing solution. Financial details of the transaction are not being disclosed. The transaction is subject to the completion of Abbott’s acquisition of Alere, as well as antitrust approvals and other customary closing conditions.
July 26, 2017 — The U.S. Senate voted Monday, with a tiebreaking vote from Vice President Mike Pence, to begin debate on ...
July 25, 2017 — In five years, Kaleida Health’s Stroke Care Center (SCC) at the Gates Vascular Institute in Buffalo, N.Y ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
July 25, 2017 — A study by researchers at the University of Birmingham has shown that general practitioners (GPs) are ...

Despite their best efforts, many patients tend to develop heart failure after an acute event (e.g., a heart attack or a ...
Using a new imaging technique that can diagnose cardiac sarcoidosis much more accurately than traditional tests, researchers from the University of Illinois at Chicago have found the disease affects other organs in 40 percent of known patients with cardiac sarcoidosis. The findings are published in the Journal of Nuclear Cardiology.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
This video case study, provided by Gore Medical, is titled "Tackling Complex Cases in Dialysis Access," by John Ross, M ...
Medtronic plc recently announced first enrollments in the STOP AF First clinical trial. The trial will evaluate the safety and effectiveness of performing pulmonary vein isolation (PVI) with the Arctic Front Advance Cryoballoon in patients with symptomatic paroxysmal atrial fibrillation (AF) prior to treatment with anti-arrhythmic medications. The first patient in the trial was recently enrolled at The Ohio State University Wexner Medical Center by Jaret Tyler, M.D.
ScImage Inc. recently announced West Virginia University Health System has partnered with ScImage to utilize ScImage’s PICOM365 Enterprise PACS (picture archiving and communication system) throughout the eight WVU Medicine hospitals. The high-security, cloud-based PICOM365 will deliver cardiovascular image management, viewing and reporting capabilities for seamless workflow throughout the health system.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
TomTec Zero is the latest addition to the TomTec portfolio. Now every physician reading cardiovascular studies at locations such as the office, reading room or from home will have full diagnostic access to all images and clinical tools, including automated strain measurements.
Samsung announced U.S. Food and Drug Administration (FDA) approval of the BodyTom Elite, an upgraded version of its portable, full-body, 32-slice computed tomography (CT) scanner. The upgraded system features a new visual design, and upgraded software, hardware and workstation.
Biotronik announced U.S. Food and Drug Administration (FDA) approval and availability of the Intica DX and Intica cardiac resynchronization therapy (CRT)-DX implantable cardioverter defibrillator (ICD) systems. The launch of Intica CRT-DX extends the benefits of Biotronik's DX technology to heart failure patients. DX eliminates the need for an atrial lead while still providing physicians with critical diagnostic information based on a true atrial signal.


 July 27, 2017
July 27, 2017