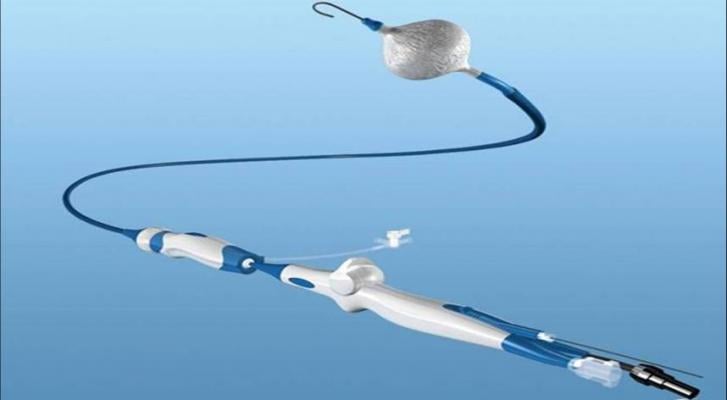
July 24, 2017 — Medtronic plc recently announced first enrollments in the STOP AF First clinical trial. The trial will evaluate the safety and effectiveness of performing pulmonary vein isolation (PVI) with the Arctic Front Advance Cryoballoon in patients with symptomatic paroxysmal atrial fibrillation (AF) prior to treatment with anti-arrhythmic medications. The first patient in the trial was recently enrolled at The Ohio State University Wexner Medical Center by Jaret Tyler, M.D.
STOP AF First is a prospective, interventional, multicenter, randomized, controlled, clinical trial that will enroll up to 210 patients at up to 30 sites in the United States. Patients will be randomized to cryoballoon ablation (treatment arm) or anti-arrhythmic drug (AAD) therapy (control arm), and followed for 12 months. Oussama Wazni, M.D., co-director of Atrial Fibrillation Center at Cleveland Clinic, serves as the study's national principal investigator.
Cryoballoon ablation is used in a minimally invasive procedure to isolate the pulmonary veins, which are a source of erratic electrical signals that cause AF. The device uses cold energy (freezing) rather than heat (radiofrequency) to create scar tissue and interrupt irregular electrical pathways in the heart.
The 2016 European Society of Cardiology's (ESC) guidelines and the recent 2017 Heart Rhythm Society (HRS) Consensus Statement for the management of atrial fibrillation both acknowledge cryoablation therapy as an appropriate ablation energy for treating AF, and recognize PVI as an effective and preferred treatment option for select patients with AF.
In the United States, first-line treatment of symptomatic paroxysmal AF with the Arctic Front Advance Cryoablation System is investigational use only; the system is approved in the U.S. for the treatment of drug refractory, recurrent, symptomatic paroxysmal AF, and in Europe for the treatment of atrial fibrillation. More than 250,000 patients in more than 50 countries worldwide have been treated with the cryoballoon.
For more information: www.medtronic.com


 February 06, 2026
February 06, 2026 









