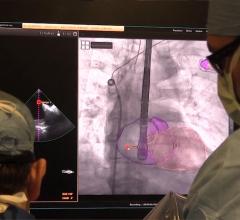
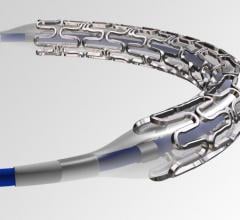
Lenox Hill Hospital is the first hospital in New York City to successfully implant Abbott’s Tendyne transcatheter mitral valve replacement (TMVR) system to replace a leaky heart valve. The procedure was performed as part of a national clinical trial testing the safety and efficacy of the device as an alternative to invasive open heart surgery.
Philips announced the latest pooled analysis of patient-level data of over 2,300 patients treated with Philips’ Stellarex Drug-Coated Balloon (DCB) in above-the-knee (ATK) studies, which the company said reinforces the strong safety profile of Stellarex. The independent, third-party pooled analysis demonstrated low mortality rates through three years after the treatment with no device-related deaths.
DAIC Editor Dave Fornell takes a tour of some of the most interesting new medical imaging technologies displayed on the ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

The anti-proliferative drug paclitaxel has been used as a coating on coronary stents to prevent restenosis since 2003 ...
January 25, 2019 — Siemens Healthineers presented its first intelligent software assistant for radiology, the AI-Rad ...
January 25, 2019 — Profusa announced promising clinical data from two studies evaluating the company's Lumee Oxygen ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Joe Cleveland, M.D., professor of cardiothoracic surgery, at the University of Colorado Hospital, offers a cardiac ...
A new report in the Journal of Vascular Surgery chronicles a multi-site randomized controlled trial comparing treatment efficacy, functional outcomes, cost effectiveness and quality of life for various treatments for critical limb ischemia (CLI). The trial will enroll 2,100 patients suffering from CLI, a debilitating and increasingly common manifestation of peripheral arterial disease (PAD) that puts patients at high risk for leg amputation, cardiovascular complications and death.
Researchers have identified another reason to limit red meat consumption: high levels of a gut-generated chemical called trimethylamine N-oxide (TMAO) that is also linked to heart disease. Scientists found that people who eat a diet rich in red meat have triple the TMAO levels of those who eat a diet rich in either white meat or mostly plant-based proteins, but discontinuation of red meat eventually lowers those TMAO levels.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...

Catheter-based blood clot removal a decade ago was a standard of care for acute coronary revascularization, but declined ...
The rise cardiovascular disease has been instrumental in fueling the coronary stent market share in the past few years ...
January 21, 2019 – Medical experts have released “Guidelines for Performing a Comprehensive Transthoracic ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
Meat-free athletes have already proven the performance-boosting power of a plant-based diet. Now, “Plant-Based Diets for Cardiovascular Safety and Performance in Endurance Sports,” a new scientific review published in the journal Nutrients1 adds further evidence that plant-based athletes benefit from improvements in heart health, performance and recovery.

Interventional cardiac resynchronization therapy (I-CRT) describes the repurposing of a set of tools and techniques ...
Abbott announced U.S. Food and Drug Administration (FDA) approval of the TactiCath Contact Force Ablation Catheter, Sensor Enabled, a new ablation catheter designed to help physicians accurately and effectively treat atrial fibrillation (AFib). The approval further expands Abbott's portfolio of cardiac ablation tools that integrate with the company's EnSite Precision cardiac mapping system to help physicians develop more precise images of the heart during cardiac ablation procedures.


 January 28, 2019
January 28, 2019