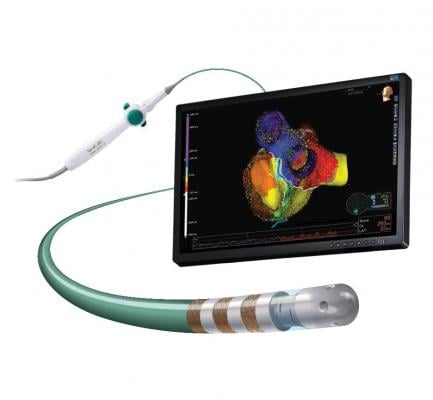
January 21, 2019 — Abbott announced U.S. Food and Drug Administration (FDA) approval of the TactiCath Contact Force Ablation Catheter, Sensor Enabled, a new ablation catheter designed to help physicians accurately and effectively treat atrial fibrillation (AFib). The approval further expands Abbott's portfolio of cardiac ablation tools that integrate with the company's EnSite Precision cardiac mapping system to help physicians develop more precise images of the heart during cardiac ablation procedures.
Similar to Abbott's other sensor-enabled mapping and ablation treatment catheters, TactiCath Sensor Enabled (SE) delivers more precise images of the heart overlaid with real-time electrical activity information. The catheter also utilizes the advanced ergonomic design found in the company's FlexAbility ablation catheter for superior reach and maneuverability during cardiac ablation procedures.
"Cardiac ablation has become a front-line therapy in the fight against conditions like atrial fibrillation in part because of improvements to the technology available today," said Roger Winkle, M.D., an electrophysiologist at Sequoia Hospital in Redwood City, Calif., and one of the first physicians to treat patients with the newly FDA-approved catheter. "The Abbott TactiCath SE ablation catheter represents one of those major improvements, and offers an incredibly powerful, yet easy to use, combination of advanced technologies to improve ablation procedures."
One of the most common cardiac arrhythmias in the world, AFib can affect how efficiently the heart pumps blood through the body, causing symptoms such as dizziness, shortness of breath or lightheadedness. The condition can also increase a person's risk of stroke. To treat conditions like AFib, physicians can use ablation catheters to scar tissue in the heart that is generating abnormal electrical signals and disrupting a patient's natural heartbeat.
Physicians have begun exploring the use of new tools such as “contact force” technology during ablation procedures to help them avoid applying too much pressure to heart tissue (resulting in complications) or insufficient pressure (which may reduce the effectiveness of the procedure). In addition, Abbott has also invested in technology to improve the accuracy of cardiac mapping to support cardiac ablation procedures.
For more information: www.abbott.com
Related Atrial Fibrillation Content
New Technologies to Improve Atrial Fibrillation Ablation
VIDEO: Current State of Atrial Fibrillation Ablation Technologies
Abbott Announces CE Mark for New Cardiac Ablation Catheter
FDA Clears St. Jude Medical Contact Force Ablation Catheter for Treatment of Atrial Fibrillation


 January 22, 2026
January 22, 2026 









