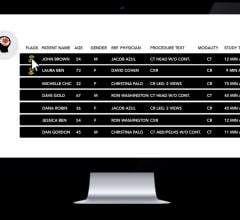
A new type of care for cardiac patients at St. Paul’s Hospital, Vancouver, has the potential to reduce heart imaging wait times, ease emergency department congestion and lower the number of unnecessary hospital admissions. At the center of the new approach is a dedicated cardiac computed tomography (CT) scanner, the first of its kind in Canada. The machine will be used as a component of a stand-alone Rapid Access Chest Pain Clinic that will provide earlier prevention and life-saving treatment to cardiac patients.
Zebra Medical Vision announced it has received its third U.S. Food and Drug Administration (FDA) 510(k) clearance for the company's HealthICH product. The new clearance covers an artificial intelligence (AI) alert for intracranial hemorrhage (ICH), based on head computed tomography (CT) scans.
DiA Imaging Analysis announced the presentation of two studies assessing the performance and accuracy of the company's LVivo EF artificial intelligence (AI)-based solution that generates fully automated left ventricular ejection fraction (LVEF). EF is a main indicator in assessing the global function of the left ventricle. Presently, many readers quantify EF visually or trace endocardial borders that may not be well defined, yielding high variability among results. LVivo EF aims to substantially reduce this variability by generating objective, accurate and fast AI-based EF.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Results from a comprehensive analysis demonstrate the effectiveness of measuring a non-hyperemic pressure ratio (NHPR), pressure distal/pressure aortic (Pd/Pa) alongside fractional flow reserve (FFR) post-percutaneous coronary intervention (PCI). This prospective study validates the diagnostic accuracy of Pd/Pa in identifying residual ischemia post-intervention against the reference standard, FFR. The study was presented at the Society for Cardiovascular Angiography and Interventions (SCAI) 2019 Scientific Sessions, May 19-22 in Las Vegas.
Philips announced the U.S. Food and Drug Administration (FDA) has approved the company’s premarket approval (PMA) application for its HeartStart OnSite defibrillator (model M5066A) and HeartStart Home defibrillator (model M5068A). The approval includes the relevant supporting accessories, such as batteries and pad electrodes.
Anthony Chang, M.D., chief artificial intelligence officer, Children’s Hospital of Orange County (CHOC), and founder ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
New study results validate the effectiveness of the Saranas Early Bird Bleed Monitoring System to sense bleeding events during endovascular-related procedures by using sensors to detect relative changes in tissue bioimpedance. The study enrolled 60 patients from five sites who underwent an endovascular procedure and detected bleeding in more than half of patients. The results of this study were presented as late- breaking clinical research on at the Society for Cardiovascular Angiography Interventions (SCAI) 2019 Scientific Sessions, May 19-22 in Las Vegas.
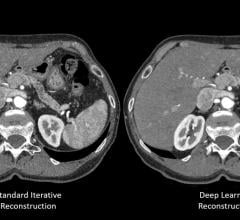
Canon Medical Systems USA Inc. has received 510(k) clearance on its new deep convolutional neural network (DCNN) image reconstruction technology, dubbed Advanced Intelligence Clear-IQ Engine (AiCE). AiCE uses a deep learning algorithm to differentiate signal from noise so that it can suppress noise while enhancing signal.
Results from a first-in-man proof of concept study found occlusion of the superior vena cava (SVC) rapidly and effectively reduces pressure and volume in the heart. This is the first study targeting the SVC as a therapeutic area for heart failure patients to reduce cardiac filling pressures without reducing cardiac output or systemic blood pressure. Data was presented as late-breaking clinical research at the Society for Cardiovascular Angiography and Interventions (SCAI) 2019 Scientific Sessions, May 19-22 in Las Vegas.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Initial results from the AVIATOR 2 international registry were presented as late-breaking clinical science at the Society for Cardiovascular Angiography and Interventions (SCAI) 2019 Scientific Sessions, May 19-22 in Las Vegas. The AVIATOR 2 is a multicenter prospective observational study of patients with atrial fibrillation (AF) undergoing percutaneous coronary intervention (PCI) in 11 international sites. The use of a novel smartphone-based survey shows potential to improve outcomes by accessing the patient need for new tools to quantify risk.
A prospective, multicenter, multinational study examines how fractional flow reserve (FFR) can impact treatment plans and outcomes in patients with stable coronary artery disease (CAD) or acute coronary syndrome (ACS). Results showed more than one-third of the patients’ initial treatment plans changed after FFR. The findings from the global registry were presented as late-breaking clinical research at the Society for Cardiovascular Angiography and Interventions (SCAI) 2019 Scientific Sessions, May 19-22 in Las Vegas.
Akshay Khandelwal, M.D., director of medical operations at the Henry Ford Heart and Vascular Institute, Detroit, and ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
Quantum Genomics announced the enrollment of the first patient in its QUORUM Phase IIb study of its lead clinical candidate, firibastat, in patients with heart failure after acute myocardial infarction (AMI). Firibastat is a first-in-class brain aminopeptidase A inhibitor, being developed for the treatment of resistant hypertension and heart failure.
June 14, 2019 – A late-breaking study examined the effects of intravascular ultrasound (IVUS) guided drug-eluting stent ...
June 14, 2019 – An expert consensus statement provides recommendations for optimizing the financial operations of the ...


 June 20, 2019
June 20, 2019