The American Society of Nuclear Cardiology (ASNC) published a new expert consensus document along with eight other societies on recommendations for multimodality imaging in cardiac amyloidosis in the Journal of Nuclear Cardiology. ASNC assembled a writing team of 26 experts in cardiovascular imaging and amyloidosis representing these nine societies: the American College of Cardiology (ACC), the American Heart Association (AHA), the American Society of Echocardiography (ASE), the European Association of Nuclear Medicine (EANM), the Heart Failure Society of America (HFSA), the International Society of Amyloidosis (ISA), the Society of Cardiovascular Magnetic Resonance (SCMR), and the Society of Nuclear Medicine and Molecular Imaging (SNMMI). Emerging imaging methods have facilitated earlier diagnosis of cardiac amyloidosis and improved prognostication and management with new treatment options. The diagnostic criteria for cardiac amyloidosis, required updating to include these novel imaging tools.

Primary percutaneous coronary intervention (PCI) is the preferred treatment for acute ST-segment elevation myocardial ...

September 9, 2019 — More than 33,000 health professionals gathered over five days to attend the 2019 European Society of ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
September 9, 2019 — The drug Dapagliflozin was found to reduce death and hospitalization in patients who have heart ...
Business-to-business communications company Scranton Gillette Communications has named Diane Vojcanin as vice president, group publisher, healthcare group. Additionally, the company is announcing that Andreja Slapsys will serve as a healthcare group integrated media consultant.

Traditionally, computed tomography (CT) and ultrasound have been the workhorse imaging modalities in the world of ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
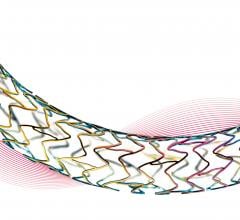
Biotronik's ultrathin Orsiro stent demonstrated superiority over Xience with respect to target lesion failure (TLF) at 12 months, according to newly released data from the BIOSTEMI trial.1 At the European Society of Cardiology (ESC) 2019 Congress, Aug. 31-Sept. 4 in Paris, France, Juan Fernando Iglesias, M.D., Ph.D., Geneva University Hospitals, Geneva, Switzerland, unveiled the results of the randomized controlled trial (RCT) in a late-breaking session. The results have also been published in The Lancet.
I recently had a great conversation with cardiac computed tomography (CT) pioneer Arthur Agatston, M.D., who is the ...
Abbott announced the launch of the company's TRILUMINATE Pivotal trial evaluating the safety and effectiveness of its TriClip transcatheter tricuspid valve repair system for the treatment of severe tricuspid regurgitation (TR). This is the first pivotal Investigational Device Exemption (IDE) trial in the U.S. to evaluate a catheter-based, non-surgical treatment for patients with severe TR – a condition in which the valve does not close properly, allowing blood to flow backward into the heart, which forces the heart to work harder. In severe cases, this condition can potentially lead to heart failure if left untreated.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
At the European Society of Cardiology (ESC) Congress 2019 together with the World Congress of Cardiology, the World Heart Federation launched a new “roadmap” aimed at reducing the global burden of CVD in people living with diabetes. The Roadmap on the prevention of cardiovascular disease among people living with diabetes is a key reference document for anyone involved in the planning, organization, implementation and monitoring, and evaluation of approaches related to CVD prevention in people living with diabetes. It outlines a vision of an ideal pathway of care, potential roadblocks along this pathway and proposed solutions, with examples from practice.
September 4, 2019 – Novartis announced results from two new clinical trials evaluating improvement in heart structure ...
September 4, 2019 — Final data from the World Alliance Societies of Echocardiography (WASE) Normal Values Study was ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
Shockwave Medical Inc. has received Breakthrough Device Designation from the U.S. Food and Drug Administration (FDA) for its Shockwave intravascular lithotripsy (IVL) system with the Shockwave C2 Coronary IVL Catheter. The device is currently the subject of an Investigational Device Exemption (IDE) study called DISRUPT CAD III. The Shockwave C2 IVL Catheter is a proprietary tool designed to fracture problematic calcium using sonic pressure waves in order to facilitate stent delivery, deployment and optimal expansion, thereby improving blood flow to the heart muscle.
Corindus Vascular Robotics Inc. announced that EClinicalMedicine, a clinical journal published by The Lancet, has published the results from the Telerobotic Intervention Study, the world’s first percutaneous coronary intervention (PCI) procedures conducted from a remote location outside the catheterization lab. The study was conducted using Corindus’ CorPath technology platform.
New Zealand-based Adept Medical announced the launch of the Antegrade IR Platform. Clinically driven, it is placed to provide an ideal work surface for antegrade femoral approach during interventional radiology vascular procedures. The device sits within the company’s existing range of access and patient positioning devices designed for interventional radiology, cardiology and vascular procedures.


 September 09, 2019
September 09, 2019