The U.S. Food and Drug Administration (FDA) has cleared the Artis icono, a high-precision family of angiography systems from Siemens Healthineers that permit a wide range of minimally invasive procedures to be performed in a single interventional suite. The Artis icono biplane system is engineered for optimal utilization in neuroradiology and abdominal imaging, while the Artis icono floor is a floor-mounted, single-plane system for vascular, interventional cardiology, surgical and oncology procedures. Both systems in the Artis icono family expand the reach of precision medicine.
September 17, 2019 — Treating high-risk heart patients with a single, high dose of radiation therapy can dramatically ...
Rex Medical L.P. has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the Revolution Peripheral Atherectomy System.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
CorWave announced successful completion of its first 60-day preclinical study to evaluate its Neptune left ventricular assist device (LVAD). The results were presented at the 46th Annual Conference of the European Society for Artificial Organs (ESAO), Sept. 8-12 in Hannover, Germany.
Corvia Medical has sponsored and is actively enrolling patients in a heart failure (HF) device trial that, in addition to measuring traditional heart failure endpoints, includes collecting and analyzing biosensor data with physIQ’s continuous remote monitoring platform. The clinical trial is designed to evaluate the clinical efficacy of Corvia’s InterAtrial Shunt Device (IASD) in patients with heart failure and is enrolling patients at more than 100 sites worldwide. Of note, the pivotal Phase 3 study design mirrors commentary within a recent U.S. Food and Drug Administration (FDA) Public Workshop and FDA Draft Guidance for Industry related to using biosensor data.
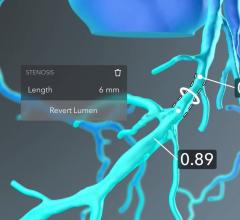
HeartFlow Inc. has obtained clearance from the U.S. Food and Drug Administration (FDA) for the HeartFlow Planner, a non-invasive, real-time virtual modeling tool for coronary artery disease (CAD) intervention. The HeartFlow Planner will enable interventional cardiologists to virtually model clinical scenarios vessel-by-vessel, explore treatment strategies for patients with CAD before each procedure, review cases with colleagues and ensure everyone has a clear picture of the initial treatment plan.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
A discussion with William O'Neill, M.D., director of the structural heart program, Henry Ford Hospital, and Michele ...
Percutaneous reduction of secondary mitral regurgitation in patients with heart failure does not lower death and hospitalization at two years compared to standard medical care, according to late breaking results from the MITRA-FR study. Results were presented in a Hot Line Session at the European Society of Cardiology (ESC) Congress 2019 together with the World Congress of Cardiology, Aug. 31-Sept. 4 in Paris, France, and published in the European Journal of Heart Failure.
The ever-present devices that seem to track all our moves can be annoying, intrusive or worse, but for heart failure patients, tiny wearable cameras could prove life-enhancing, according to new research. The research was presented at the European Society of Cardiology (ESC) Congress 2019, Aug. 31-Sept. 4 in Paris, France, together with the World Congress of Cardiology.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
The angiotensin neprilysin inhibitor sacubitril/valsartan (Entresto) missed its primary endpoint of reducing total hospitalization and cardiovascular death in patients with heart failure with preserved ejection fraction (HFpEF), but the data suggest there may be benefit in some patient groups. The late-breaking results of the PARAGON-HF trial were presented in a Hot Line Session at the European Society of Cardiology (ESC) Congress 2019, Aug. 31-Sept. 4 in Paris, France, together with the World Congress of Cardiology and published in the New England Journal of Medicine.
Scott Schwartz, M.D., interventional radiologist and program director for IR residencies and the vascular and ...
The combination of ticagrelor and aspirin reduces ischemic events compared with aspirin alone in patients with stable coronary artery disease and diabetes. The late-breaking results of the THEMIS trial were presented in a Hot Line Session at the European Society of Cardiology (ESC) Congress 2019, Aug. 31-Sept. 4 in Paris, France, together with the World Congress of Cardiology and published in the New England Journal of Medicine.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
An international randomized trial has shown that complete revascularization reduces major cardiovascular events compared to culprit-lesion only percutaneous coronary intervention (PCI). Late breaking results of the COMPLETE trial were presented in a Hot Line Session at the European Society of Cardiology (ESC) Congress 2019 together with the World Congress of Cardiology, and published in the New England Journal of Medicine.
Prasugrel is superior to ticagrelor for reducing ischemic events in patients with acute coronary syndrome and a planned invasive strategy. The late-breaking results of the ISAR-REACT 5 trial were presented in a Hot Line Session at the European Society of Cardiology (ESC) Congress 2019, Aug. 31-Sept. 4 in Paris, France together with the World Congress of Cardiology and published in the New England Journal of Medicine.[1] There was no increase in the rate of major bleeding with prasugrel.
AstraZeneca announced detailed results from the landmark Phase III DAPA-HF trial that showed Farxiga (dapagliflozin) on top of standard of care reduced both the incidence of cardiovascular (CV) death and the worsening of heart failure.

 September 17, 2019
September 17, 2019