August 15, 2022 — Researchers in the Biomedical Engineering Department at UConn have developed a new cardiac cell-derived platform that closely mimics the human heart, unlocking potential for more thorough preclinical drug development and testing, and model for cardiac diseases.
The research, published in Cell Reports by Assistant Professor Kshitiz in collaboration with Dr. Junaid Afzal in the cardiology department at the University of California San Francisco, presents a method that accelerates maturation of human cardiac cells towards a state suitable enough to be a surrogate for preclinical drug testing.
“There is a very strong need to create human cardiac constructs for all sorts of applications. Small animal models just do not recapitulate human heart biology, and human samples are scarce,” says Kshitiz. “This matters because all drugs need to be tested for their toxicity to heart. It is widely believed that a large number of them unnecessarily fail clinical trials because we do not have human samples to test them with.”
Kshitiz and Afzal first identified the need to create a matured human cardiac tissue during their time together at Johns Hopkins Medicine.
“When methods were developed to differentiate human pluripotent stem cells to cardiac cells, it created a big hope that finally we will have human heart constructs to work with,” said Afzal. “While it is straightforward to get human cardiac cells, they are similar to fetal cells. What we need is adult cells.”
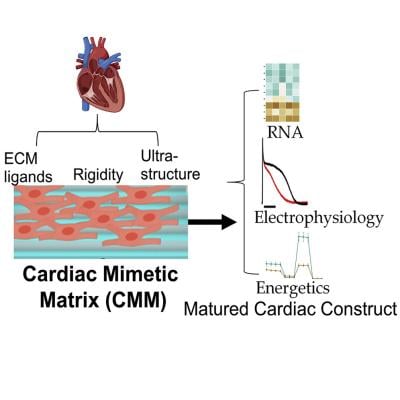
Cardiovascular safety is the number one cause for failure of preclinical drug development, and there is a long standing need to create human cardiac tissue models to test drugs for cardiotoxicity. Currently, the small animal heart models display vastly different biochemical, physiological, and genetic features from humans—making it difficult to replicate the human heart in preclinical studies. In particular, it is very difficult to perform metabolic assessment of current cardiac constructs. Heart beats continuously and is a highly metabolically active organ.
“Metabolic and redox maturation is critical for heart cells, and we are able to achieve it and possibly create a gold standard—fundamentally shifting our expectations of creating a metabolically mature cardiac tissue,” the researchers said.
In the study, the researchers utilized the cardiac biology in an adult human heart to rapidly mature differentiated cardiac cells into a more adult-like state. Within 30 days, the researchers achieved cardiac cells that displayed structural, mechanical, metabolic, and electrophysiological characteristics close to adult heart muscle.
The researchers are optimistic that this application will not only be used for preclinical drug testing, but can also be used in future precision disease modeling to study disease mechanisms and test for regenerative therapies. The researchers hope that the many drugs that fail unnecessarily due to non-human methods will be salvaged for cures for cancer, immune and neurological diseases.
For more information: https://health.uconn.edu/


 January 05, 2026
January 05, 2026 









