January 16, 2019 — Cerenovus, of Johnson & Johnson Medical Devices Companies, recently launched the EXCELLENT Registry to collect and analyze stroke-inducing blood clots removed from the brain with its Embotrap II Revascularization Device. The company also announced European CE Mark approval for the Geometric Clot Extractor (GCE) Revascularization Device.
The EXCELLENT Registry, which the company calls the single largest global registry of its kind, will enroll up to 1,000 ischemic stroke patients in as many as 50 clinical sites in the United States and Europe. Real-world evidence will be collected on all patients, and clots will be preserved and studied to determine how different clot characteristics — including size, composition and density — may impact or relate to patient comorbidities, clinical outcomes and revascularization rates.
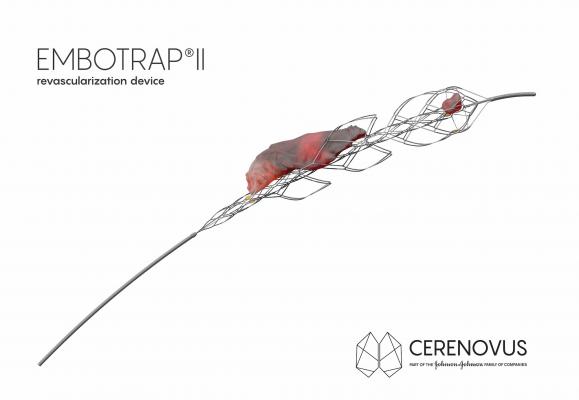
Extensive clot research conducted by Cerenovus was the basis for the development of the Embotrap II Device, a next-generation stent retriever that features a dual-layer design that allows doctors to maintain engagement and control of a broad range of clots with minimal compression during removal. The device was cleared by the U.S. Food and Drug Administration (FDA) earlier in 2018 and has been available in Europe since 2016.
While advances in thrombectomy have made it standard practice, about 20 percent or more of ischemic stroke cases remain resistant to the procedure due to the nature and the composition of certain blood clots.[1] In an effort to fill this clinical need, Cerenovus has developed the GCE Revascularization Device, a novel stroke technology shown to retrieve various thrombus types, whether fibrin-rich thrombus (hard) or RBC-rich thrombus (soft). The company received CE mark approval in the European Union for the device in December and is conducting a controlled evaluation study prior to its commercial launch to assess its clinical utility and potential advantages over existing technologies.
Strokes strike nearly 800,000 Americans each year, and is a leading cause of disability, and the fifth leading cause of death.[2] For stroke patients, the difference between life and death, and between functional independence and disability, is tied to timely restoration of blood flow to the oxygen-starved brain after a stroke event occurs.[3] In the U.S. alone, the economic burden of stroke is estimated at $34 billion annually, which includes the cost of health care services, medications, and lost productivity.[4]
For more information: www.cerenovus.com
References
[1] Fennell VS, et al. J NeuroIntervent Surg 2018;0:1–4. doi:10.1136/neurintsurg-2017-013507
[2] American Heart Association "Impact of Stroke (Stroke Statistics)" http://www.strokeassociation.org/STROKEORG/AboutStroke/Impact-of-Stroke-Stroke-statistics_UCM_310728_Article.jsp#.WmDsuSOZMQ8 Date Accessed: 03 December 2018.
[3] Mayo Foundation for Medical Education and Research (MFMER) "Stroke" https://www.mayoclinic.org/diseases-conditions/stroke/diagnosis-treatment/drc-20350119 Date Accessed: 04 December 2018.
[4] Centers for Disease Control "Stroke Fact Sheet" https://www.cdc.gov/dhdsp/data_statistics/fact_sheets/fs_stroke.htm Date Accessed: 03 December 2018.


 January 05, 2026
January 05, 2026 









