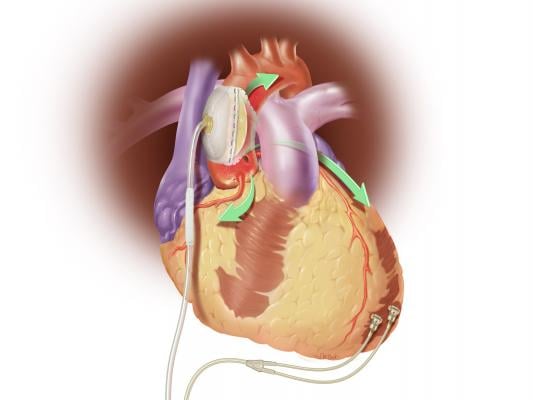
February 25, 2015 — Sunshine Heart Inc. announced it has received unconditional approval from the U.S. Food and Drug Administration (FDA) to conduct an interim analysis of COUNTER HF, the company's U.S. pivotal study. The COUNTER HF study is a prospective, randomized, multi-center, controlled study that evaluates the safety and efficacy of the C-Pulse system for the treatment of NYHA Class III and ambulatory Class IV heart failure. Integral to the COUNTER HF study is the assessment of C-Pulse's balloon counterpulsation treatment designed to improve heart function and reduce re-hospitalizations due to worsening heart failure.
A key potential benefit of conducting the analysis is the prospect of reducing the overall duration of the study should COUNTER HF meet the higher statistical threshold of the interim analysis. The study is a prospective, randomized, multi-center clinical trial. It is being conducted by heart failure and cardiac surgeon specialists in the United States and is expected to randomize 388 patients in up to 40 clinical sites. The purpose of the study is to determine whether the C-Pulse System is a safe and effective treatment for heart failure patients who meet the following key study qualifications:
- NYHA Class III or early Class IV heart failure;
- Ejection fraction less than or equal to 35 percent (measure of how well the heart pumps blood);
- Taking appropriate heart failure medications as prescribed by doctor; and
- Have been evaluated for cardiac resynchronization therapy with or without defibrillation (CRT, CRT-D) or implantable cardioverter defibrillator (ICD) therapy.
The C-Pulse Heart Assist System utilizes the scientific principles of intra-aortic balloon counter-pulsation applied in an extra-aortic approach to assist the left ventricle by reducing the workload required to pump blood throughout the body, while increasing blood flow to the coronary arteries. Combined, these potential benefits may help sustain the patient's current condition or, in some cases, reverse the heart failure process, thereby potentially preventing the need for later-stage heart failure devices, such as left ventricular assist devices (LVADs), artificial hearts or transplants. It may also provide relief from the symptoms of Class III and ambulatory Class IV heart failure and improve quality of life and cardiac function.
For more information: www.hfclinicalstudy.com


 January 05, 2026
January 05, 2026 









