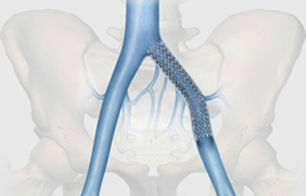
September 3, 2014 — Jobst Vascular Institute is conducting pioneering research that looks at using stents to treat blocked veins. For years, stents have been used to treat narrowed or blocked arteries; however none are specifically designed for use in veins. The Cook Medical VIVO clinical research study aims to determine the safety and effectiveness of the Zilver vena venous self-expanding stent in the treatment of vein obstructions. Jobst Vascular Institute is the second center in the country to enroll a patient in the VIVO clinical research study.
“Veins differ greatly from arteries and require a different approach when treating blockages,” said Anthony Comerota, M.D., one of two global principal investigators in the VIVO clinical research study. “While arteries are muscular and under high pressure as they deliver blood throughout the body, veins are low-pressure vessels that return blood to the heart. When obstructed, they require stent characteristics different from those required by an artery.”
Vein obstruction or iliofemoral venous outflow obstruction is a blockage or narrowing of the major veins draining blood from the leg. It causes leg pain, throbbing, swelling and skin discoloration in the legs. It may also be associated with deep vein thrombosis, a blood clot in the leg. Patients are currently treated only with compression stockings or by elevating the leg. Some patients may be taking blood thinners.
“This is an important trial, which may expand the options for treating diseased veins,” said Comerota.
The Zilver vena venous self-expanding stent is an investigational device that is not approved for sale in the United States.
For more information: www.promedica.org/jobst, www.cookmedical.com


 January 05, 2026
January 05, 2026 









